|
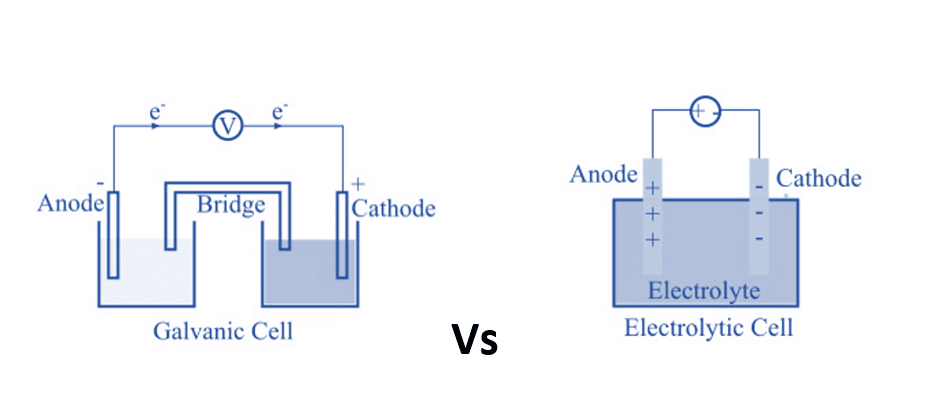
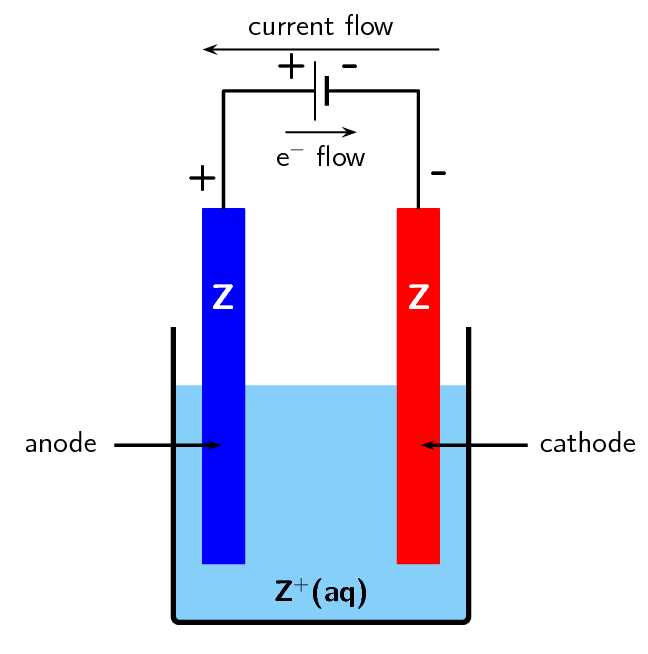
In a galvanic cell, oxidation occurs at the ___ and reduction occurs at the ___. |
Card: 3 / 52 |
|
It states that the amount of substance deposited at an electrode is directly proportional to the charge passed through the solution. |
Card: 8 / 52 |
|
Fill in the blank: In electrolysis, the anode is the ___ electrode where oxidation occurs. |
Card: 9 / 52 |
|
True or False: The electromotive force (EMF) of a cell must be negative for the reaction to be feasible. |
Card: 11 / 52 |
|
It relates Gibbs free energy to cell potential and allows for the calculation of cell potential under non-standard conditions. |
Card: 14 / 52 |
|
What is the significance of the standard hydrogen electrode in electrochemistry? |
Card: 23 / 52 |
|
It serves as a reference electrode to determine the electrode potentials of other half-cells. |
Card: 24 / 52 |
|
Riddle: I am the process of breaking down compounds using electricity. What am I? |
Card: 25 / 52 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
In an electrolytic cell, the electrolyte provides a medium for ___ between electrodes. |
Card: 27 / 52 |
|
The extra voltage needed to drive a non-spontaneous reaction beyond the theoretical voltage. |
Card: 30 / 52 |
|
Temperature variations can influence the kinetic energy of the reactants and the overall Gibbs free energy, thus affecting cell potential. |
Card: 32 / 52 |
|
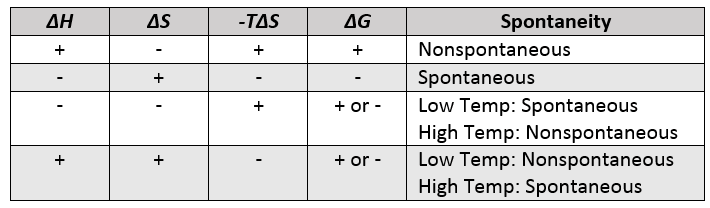
What is the relationship between Gibbs free energy and spontaneity of a reaction? |
Card: 33 / 52 |
|
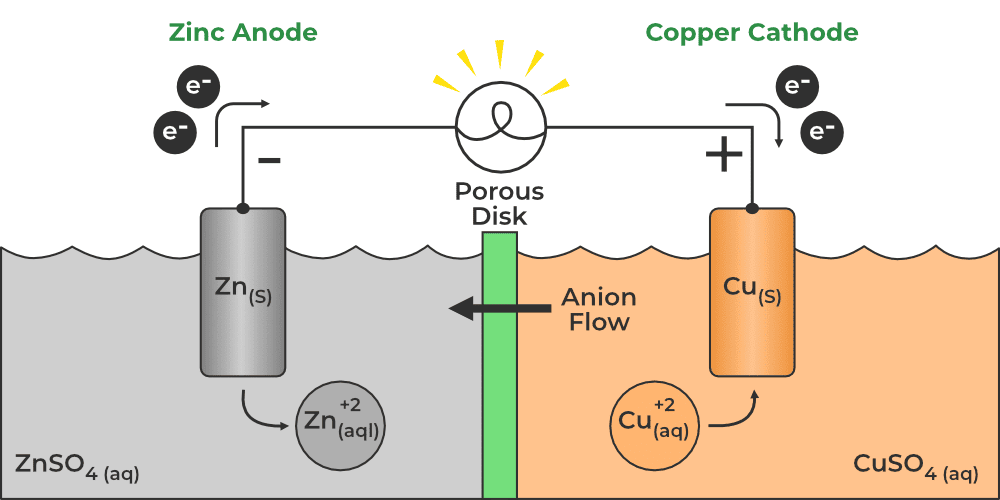
An electrochemical cell is a device that converts chemical energy into electrical energy or vice versa. |
Card: 40 / 52 |
|
Eºcell = Standard reduction potential of cathode - Standard reduction potential of anode. |
Card: 46 / 52 |
|
True or False: Electrode potentials are assessed in relation to a standard reference electrode to determine the feasibility of reactions. |
Card: 47 / 52 |
|
The equivalent weight is the mass of a substance that reacts with or produces one mole of hydrogen ions (H+). |
Card: 50 / 52 |
|
Riddle: I am the unit charge that can liberate one gram equivalent of a substance at an electrode. What am I? |
Card: 51 / 52 |