|
The general formula for carbonyl compounds is ___ where n represents the number of carbon atoms. |
Card: 3 / 44 |
|
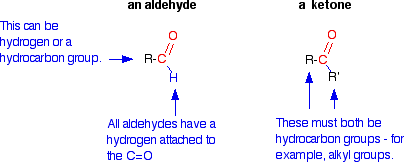
In the context of carbonyl compounds, what distinguishes aldehydes from ketones? |
Card: 5 / 44 |
|
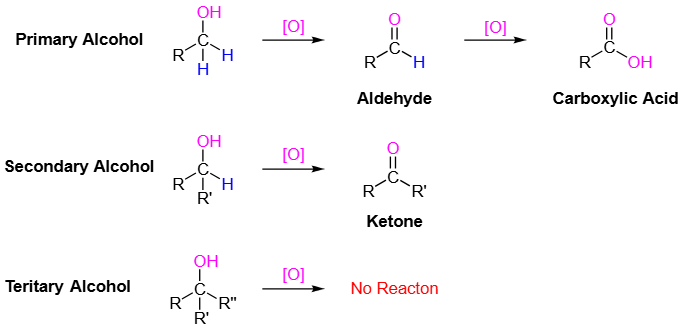
Aldehydes have the carbonyl group bonded to at least one hydrogen atom, while ketones have the carbonyl group bonded between two carbon atoms. |
Card: 6 / 44 |
|
The IUPAC naming convention for aldehydes ends with the suffix ___ and for ketones with ___ . |
Card: 9 / 44 |
|
True or False: Cyclic ketones are numbered starting from the carbon atom furthest from the carbonyl group. |
Card: 11 / 44 |
|
Fill in the blank: The carbonyl group exhibits ___ hybridization, leading to a trigonal coplanar structure. |
Card: 13 / 44 |
|
The carbonyl oxygen acts like a Lewis base due to its two unshared pairs of electrons. |
Card: 16 / 44 |
|
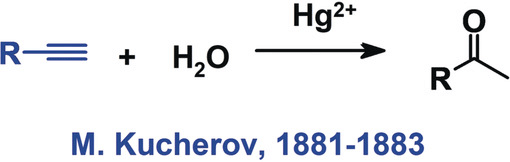
It adds water to the carbon-carbon triple bond, forming a carbonyl compound (ketone or aldehyde). |
Card: 22 / 44 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
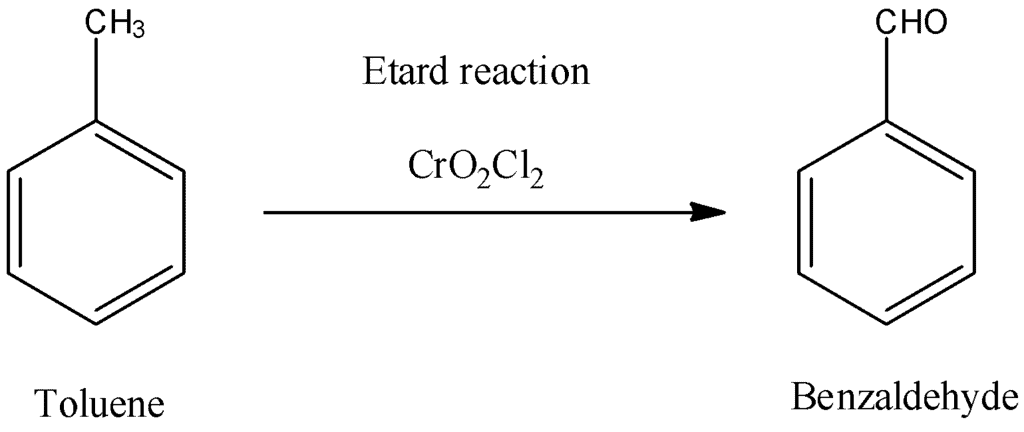
The Etard reaction uses ___ as an oxidizing agent to convert toluene into benzaldehyde. |
Card: 23 / 44 |
|
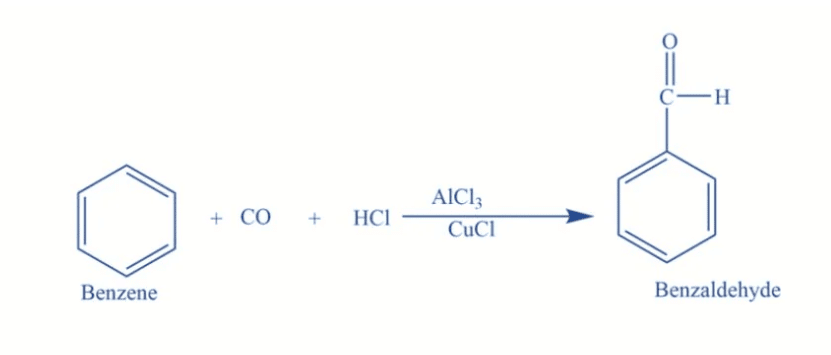
The Gattermann-Koch reaction introduces a formyl group (-CHO) to an aromatic compound using ___ and ___. |
Card: 25 / 44 |
|
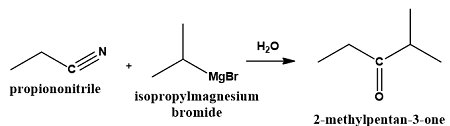
What is produced when a nitrile is treated with a Grignard reagent followed by hydrolysis? |
Card: 27 / 44 |
|
Fill in the blank: The carbonyl carbon in a carbonyl group has ___ sigma bonds and one ___ bond. |
Card: 29 / 44 |
|
The hydroboration-oxidation reaction can be used to convert alkenes into ___ if the starting material is an alkene. |
Card: 33 / 44 |
|
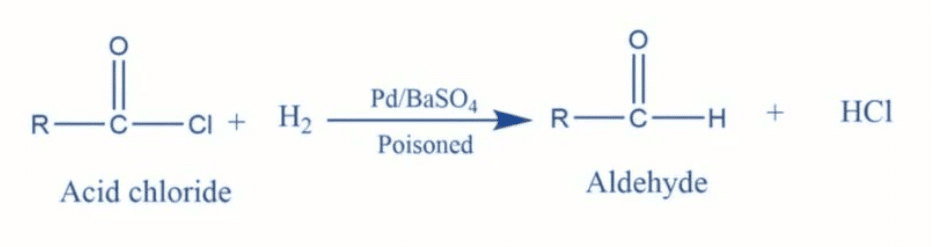
What are common reducing agents used in the reduction of acyl halides, esters, and nitriles to form aldehydes? |
Card: 37 / 44 |
|
Fill in the blank: The polarization in the carbon-oxygen bond of carbonyl compounds is due to the higher electronegativity of ___ compared to ___. |
Card: 39 / 44 |