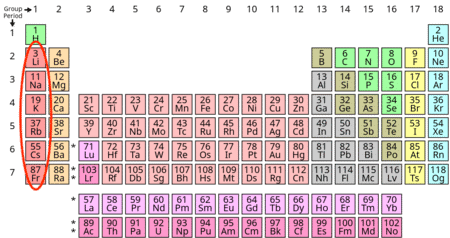|
Moseley's Periodic Law states that the physical and chemical properties of elements are periodic functions of their ___ . |
Card: 1 / 40 |
|
Which group of elements have the same electronic configuration of ns¹, leading to similar properties? |
Card: 3 / 40 |
|
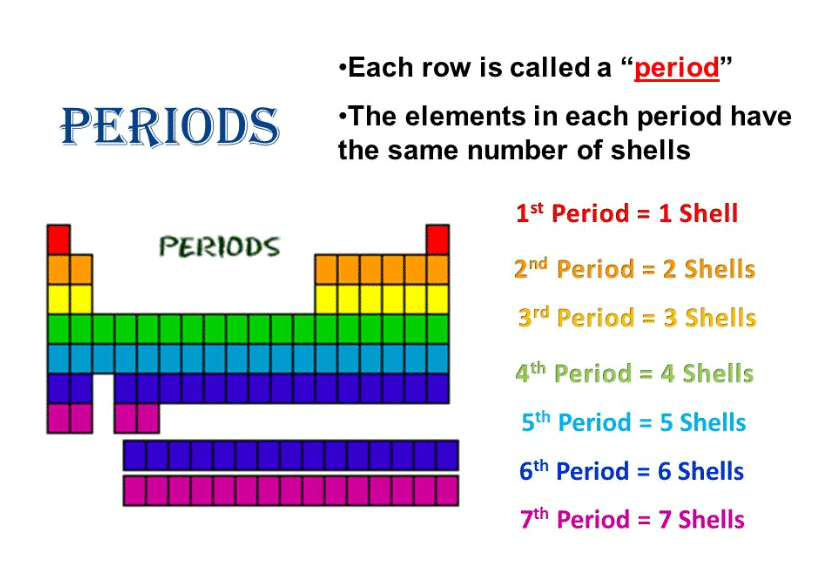
Identify the general trend in the number of elements present in each period of the periodic table. |
Card: 5 / 40 |
|
The number of elements in each period is twice the number of atomic orbitals available in the energy level being filled. |
Card: 6 / 40 |
|
Noble gases are chemically inert.
|
Card: 8 / 40 |
|
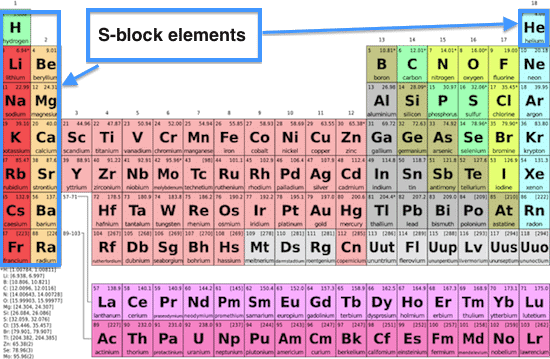
True or False: The last entered electron determines the block of an element in the periodic table. |
Card: 11 / 40 |
|
Riddle: I am a gas that is colorless and inert, with eight electrons in my outer shell. What am I? |
Card: 17 / 40 |
|
Metalloids have properties intermediate between metals and nonmetals and include elements like Boron, Silicon, and Germanium. |
Card: 20 / 40 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Fill in the blank: The long form of the periodic table is also known as ___ table. |
Card: 21 / 40 |
|
Periodic properties arise from the recurrence of similar valence shell electronic configurations at regular intervals. |
Card: 26 / 40 |
|
Riddle: I am a liquid non-metal at room temperature that belongs to the halogens. What am I? |
Card: 29 / 40 |
|
First and third periods differ in element count.
|
Card: 32 / 40 |
|
What is the role of the principal quantum number in defining periods of the periodic table? |
Card: 37 / 40 |
|
The principal quantum number corresponds to the energy level of the valence shell, determining which period an element belongs to. |
Card: 38 / 40 |
|
Describe the significance of the Aufbau Principle regarding element classification. |
Card: 39 / 40 |
|
The Aufbau Principle aids classification.
|
Card: 40 / 40 |