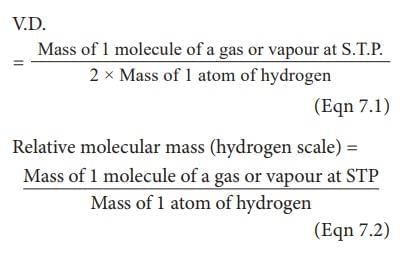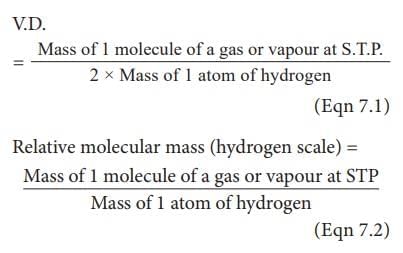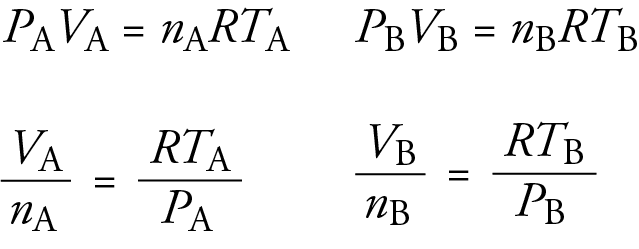|
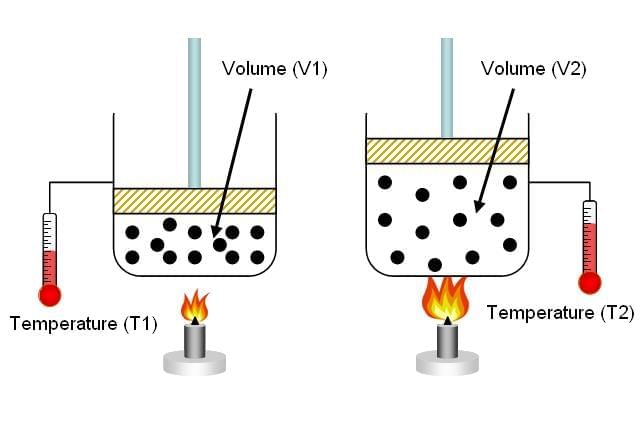
According to Boyle’s Law, if the pressure of a gas is doubled, what happens to its volume? |
Card: 1 / 30 |
 The volume of the gas is halved, since pressure and volume are inversely proportional. |
Card: 2 / 30 |
|
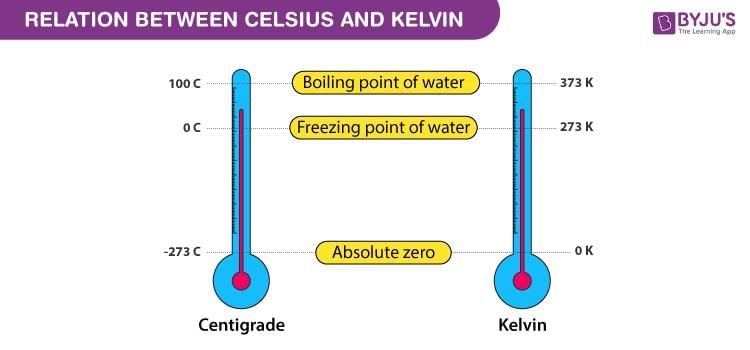
Fill in the blank: When the absolute temperature of a gas increases at constant pressure, its volume ___ . |
Card: 3 / 30 |
|
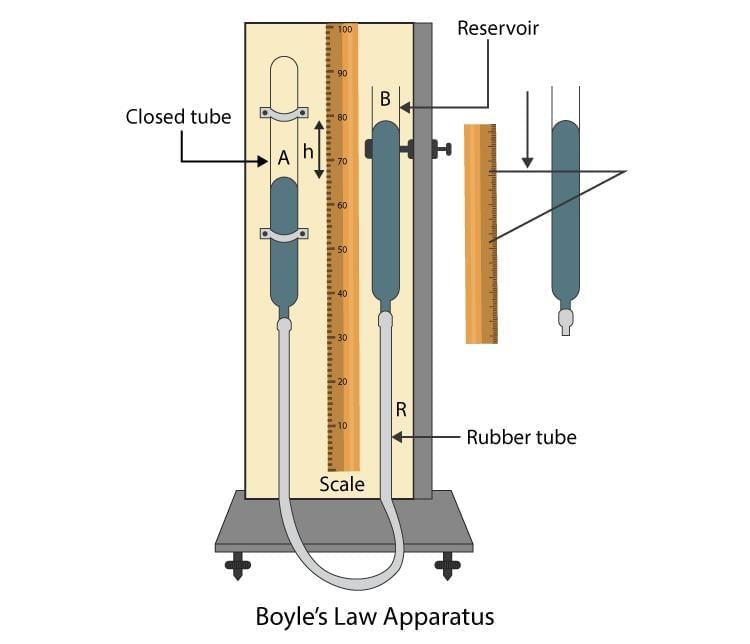
True or False: Gay-Lussac’s law states that the volume of a gas is directly proportional to its temperature at constant pressure. |
Card: 7 / 30 |
 False; it states that pressure is directly proportional to temperature at constant volume. |
Card: 8 / 30 |
|
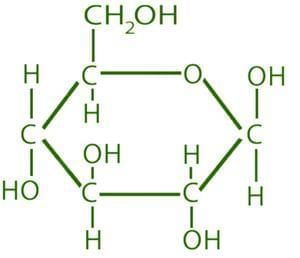
What is the molecular formula for a gas if its empirical formula is CH₂ and its molar mass is 42 g/mol? |
Card: 11 / 30 |
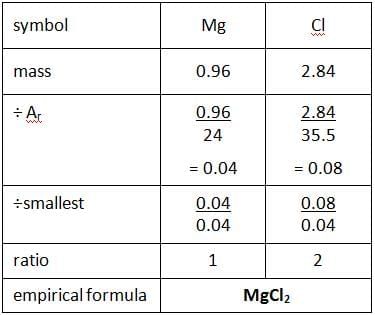 C₃H₆, since the empirical formula mass is 14 g/mol, and 42 g/mol / 14 g/mol = 3. |
Card: 12 / 30 |
|
Fill in the blank: Avogadro’s Law states that equal volumes of gases at the same temperature and pressure contain ___ . |
Card: 13 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
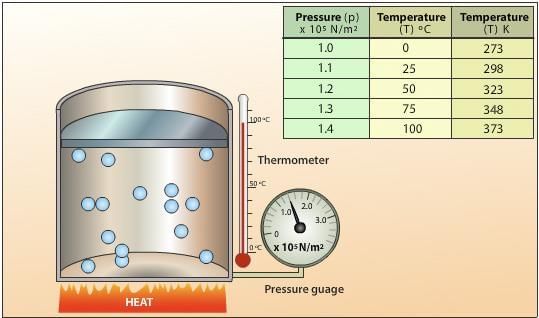
What is the relationship between molecular mass and vapor density as per the gas laws? |
Card: 19 / 30 |
|
Fill in the blank: The ratio of reacting gases according to Gay-Lussac’s law can be expressed in simple ___ . |
Card: 23 / 30 |
|
If the empirical formula of a compound is NH₂ and its molecular formula has a molar mass of 32 g/mol, what is the molecular formula? |
Card: 27 / 30 |
 N₂H₄, since the empirical formula mass is 16 g/mol and 32 g/mol / 16 g/mol = 2. |
Card: 28 / 30 |
|
True or False: The empirical formula provides the exact number of atoms of each element in a molecule. |
Card: 29 / 30 |
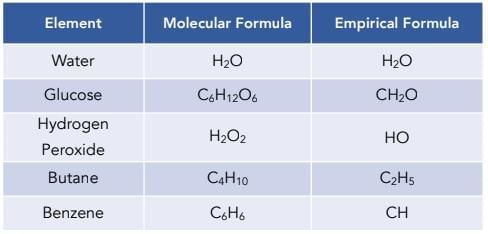 False; the empirical formula shows the simplest ratio of elements in a compound. |
Card: 30 / 30 |