Class 12 Exam > Class 12 Questions > What is the structure of the compound 3-ethyl...
Start Learning for Free
What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.?
Most Upvoted Answer
What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl p...
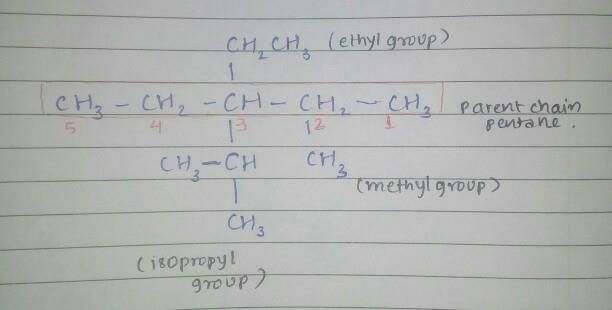
Community Answer
What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl p...
Structure of 3-ethyl-3-isopropyl-2-methylpentane
To understand the structure of the compound 3-ethyl-3-isopropyl-2-methylpentane, let's break it down step by step.
1. Identify the parent chain:
The compound contains five carbon atoms, so the parent chain will have five carbons. Since it is a pentane derivative, the parent chain is a pentane.
2. Identify and locate the substituents:
The compound has three substituents: ethyl, isopropyl, and methyl. Let's locate each of them on the parent chain:
- The ethyl group is located at the third carbon atom.
- The isopropyl group is located at the third carbon atom as well.
- The methyl group is located at the second carbon atom.
3. Number the parent chain:
Number the parent chain starting from the end that gives the lowest possible numbers to the substituents. In this case, numbering from the right end gives the lowest numbers for all substituents.
4. Name and position the substituents:
Using the numbering, we can now name and position the substituents:
- The ethyl group is located at the 3rd carbon, so it is named as 3-ethyl.
- The isopropyl group is also located at the 3rd carbon, so it is named as 3-isopropyl.
- The methyl group is located at the 2nd carbon, so it is named as 2-methyl.
5. Combine the names:
Combine the names of the substituents in alphabetical order, separated by hyphens, with the parent chain name at the end:
3-ethyl-3-isopropyl-2-methylpentane
6. Structural representation:
The structural representation of 3-ethyl-3-isopropyl-2-methylpentane can be drawn as follows:
CH3 CH3
| |
CH3 - C - C - C - C - CH2 - CH3
| |
CH3 CH2CH3
In this structure, the five carbon atoms of the pentane chain are represented by horizontal lines, and the substituents are attached to the appropriate carbon atoms.
Summary:
The compound 3-ethyl-3-isopropyl-2-methylpentane consists of a pentane parent chain with three substituents: ethyl, isopropyl, and methyl. The substituents are located at the 3rd carbon atom (ethyl and isopropyl) and the 2nd carbon atom (methyl). The IUPAC name of the compound is derived by combining the names of the substituents in alphabetical order with the parent chain name, resulting in 3-ethyl-3-isopropyl-2-methylpentane.
To understand the structure of the compound 3-ethyl-3-isopropyl-2-methylpentane, let's break it down step by step.
1. Identify the parent chain:
The compound contains five carbon atoms, so the parent chain will have five carbons. Since it is a pentane derivative, the parent chain is a pentane.
2. Identify and locate the substituents:
The compound has three substituents: ethyl, isopropyl, and methyl. Let's locate each of them on the parent chain:
- The ethyl group is located at the third carbon atom.
- The isopropyl group is located at the third carbon atom as well.
- The methyl group is located at the second carbon atom.
3. Number the parent chain:
Number the parent chain starting from the end that gives the lowest possible numbers to the substituents. In this case, numbering from the right end gives the lowest numbers for all substituents.
4. Name and position the substituents:
Using the numbering, we can now name and position the substituents:
- The ethyl group is located at the 3rd carbon, so it is named as 3-ethyl.
- The isopropyl group is also located at the 3rd carbon, so it is named as 3-isopropyl.
- The methyl group is located at the 2nd carbon, so it is named as 2-methyl.
5. Combine the names:
Combine the names of the substituents in alphabetical order, separated by hyphens, with the parent chain name at the end:
3-ethyl-3-isopropyl-2-methylpentane
6. Structural representation:
The structural representation of 3-ethyl-3-isopropyl-2-methylpentane can be drawn as follows:
CH3 CH3
| |
CH3 - C - C - C - C - CH2 - CH3
| |
CH3 CH2CH3
In this structure, the five carbon atoms of the pentane chain are represented by horizontal lines, and the substituents are attached to the appropriate carbon atoms.
Summary:
The compound 3-ethyl-3-isopropyl-2-methylpentane consists of a pentane parent chain with three substituents: ethyl, isopropyl, and methyl. The substituents are located at the 3rd carbon atom (ethyl and isopropyl) and the 2nd carbon atom (methyl). The IUPAC name of the compound is derived by combining the names of the substituents in alphabetical order with the parent chain name, resulting in 3-ethyl-3-isopropyl-2-methylpentane.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.?
Question Description
What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.?.
What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.?.
Solutions for What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? defined & explained in the simplest way possible. Besides giving the explanation of
What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.?, a detailed solution for What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? has been provided alongside types of What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? theory, EduRev gives you an
ample number of questions to practice What is the structure of the compound 3-ethyl-3- isopropyl-2- methyl pentane.? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















