Class 11 Exam > Class 11 Questions > What distinction between 'metals'and 'non-met...
Start Learning for Free
What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ?
Most Upvoted Answer
What distinction between 'metals'and 'non-metals'on the basis of follo...
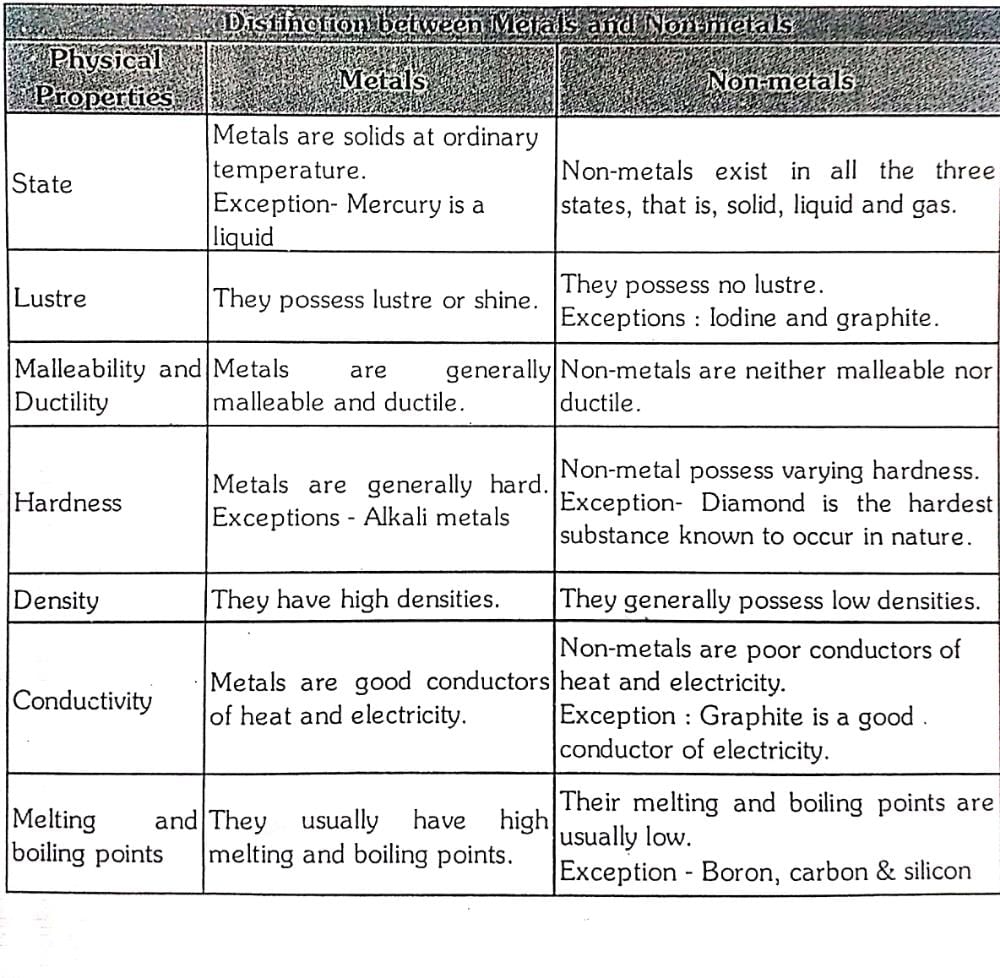
Community Answer
What distinction between 'metals'and 'non-metals'on the basis of follo...
**Metals vs Non-metals: Physical Properties**
Metals and non-metals are two distinct categories of elements based on their physical and chemical properties. In this response, we will discuss the distinction between metals and non-metals based on various physical properties.
**Existence:**
- Metals: Most metals are solid at room temperature, with a few exceptions like mercury.
- Non-metals: Non-metals can exist in all three states of matter - solid, liquid, and gas. Examples include oxygen (gas), sulfur (solid), and bromine (liquid).
**Luster:**
- Metals: Metals generally have a shiny and reflective surface, known as metallic luster.
- Non-metals: Non-metals lack metallic luster and are generally dull or have a glassy appearance.
**Hardness:**
- Metals: Metals tend to be hard, although there are exceptions like sodium and potassium, which are soft enough to be cut with a knife.
- Non-metals: Non-metals have varying degrees of hardness. Some non-metals like diamond are extremely hard, while others like sulfur are relatively soft.
**Density:**
- Metals: Metals are generally dense materials. For example, iron and lead have high densities.
- Non-metals: Non-metals have lower densities compared to metals. For instance, gases like hydrogen and helium have very low densities.
**Conductivity:**
- Metals: Metals are good conductors of heat and electricity. They possess a high degree of metallic bonding that allows the movement of electrons, leading to their excellent conductivity.
- Non-metals: Non-metals are generally poor conductors of heat and electricity. They lack the free-moving electrons required for efficient conduction.
**Malleability and Ductility:**
- Metals: Metals are malleable, meaning they can be hammered or pressed into different shapes without breaking. They are also ductile, which means they can be drawn into thin wires.
- Non-metals: Non-metals are generally brittle and cannot be easily shaped or stretched without breaking.
In conclusion, metals and non-metals can be distinguished based on several physical properties. Metals exist primarily as solids, have metallic luster, are generally hard and dense, exhibit high conductivity, and possess malleability and ductility. On the other hand, non-metals can exist in all states, lack metallic luster, have varying degrees of hardness and density, are poor conductors of heat and electricity, and are generally brittle. Understanding these distinctions is essential for categorizing and studying the properties of different elements in the periodic table.
Metals and non-metals are two distinct categories of elements based on their physical and chemical properties. In this response, we will discuss the distinction between metals and non-metals based on various physical properties.
**Existence:**
- Metals: Most metals are solid at room temperature, with a few exceptions like mercury.
- Non-metals: Non-metals can exist in all three states of matter - solid, liquid, and gas. Examples include oxygen (gas), sulfur (solid), and bromine (liquid).
**Luster:**
- Metals: Metals generally have a shiny and reflective surface, known as metallic luster.
- Non-metals: Non-metals lack metallic luster and are generally dull or have a glassy appearance.
**Hardness:**
- Metals: Metals tend to be hard, although there are exceptions like sodium and potassium, which are soft enough to be cut with a knife.
- Non-metals: Non-metals have varying degrees of hardness. Some non-metals like diamond are extremely hard, while others like sulfur are relatively soft.
**Density:**
- Metals: Metals are generally dense materials. For example, iron and lead have high densities.
- Non-metals: Non-metals have lower densities compared to metals. For instance, gases like hydrogen and helium have very low densities.
**Conductivity:**
- Metals: Metals are good conductors of heat and electricity. They possess a high degree of metallic bonding that allows the movement of electrons, leading to their excellent conductivity.
- Non-metals: Non-metals are generally poor conductors of heat and electricity. They lack the free-moving electrons required for efficient conduction.
**Malleability and Ductility:**
- Metals: Metals are malleable, meaning they can be hammered or pressed into different shapes without breaking. They are also ductile, which means they can be drawn into thin wires.
- Non-metals: Non-metals are generally brittle and cannot be easily shaped or stretched without breaking.
In conclusion, metals and non-metals can be distinguished based on several physical properties. Metals exist primarily as solids, have metallic luster, are generally hard and dense, exhibit high conductivity, and possess malleability and ductility. On the other hand, non-metals can exist in all states, lack metallic luster, have varying degrees of hardness and density, are poor conductors of heat and electricity, and are generally brittle. Understanding these distinctions is essential for categorizing and studying the properties of different elements in the periodic table.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ?
Question Description
What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ?.
What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ?.
Solutions for What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? defined & explained in the simplest way possible. Besides giving the explanation of
What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ?, a detailed solution for What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? has been provided alongside types of What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? theory, EduRev gives you an
ample number of questions to practice What distinction between 'metals'and 'non-metals'on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























