Class 12 Exam > Class 12 Questions > what is ment by positional isomerism please e...
Start Learning for Free
what is ment by positional isomerism please explain with example
Verified Answer
what is ment by positional isomerism please explain with example
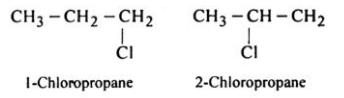
Position Isomerism:-The positions of the functional groups or substituent atoms are different in position isomers.
Typically, this isomerism involves the attachment of the functional groups to different carbon atoms in the carbon chain.
An example of this type of isomerism can be observed in the compounds having the formula C3H7Cl.
 This question is part of UPSC exam. View all Class 12 courses
This question is part of UPSC exam. View all Class 12 courses
Most Upvoted Answer
what is ment by positional isomerism please explain with example
Positional isomers arises due to different positions of side chains,substitutents,functional groups and bonds on parent chain.
CH3-CH2-CH2-Cl. CH3-CH-CH3
|
Cl
CH2=CH-CH2-CH3 CH3-CH=CH-CH3
CH3-CH2-CH2-OH. CH3-CH-CH3
I
OH
CH3-CH2-CH2-Cl. CH3-CH-CH3
|
Cl
CH2=CH-CH2-CH3 CH3-CH=CH-CH3
CH3-CH2-CH2-OH. CH3-CH-CH3
I
OH
Community Answer
what is ment by positional isomerism please explain with example
Positional isomerism refers to a type of structural isomerism in organic chemistry where compounds have the same molecular formula but differ in the position of functional groups or substituents on the carbon skeleton. In other words, positional isomers have the same atoms but arranged differently.
Example:
Let's consider the compound C4H9Br, which can exhibit positional isomerism. In this case, the bromine atom (Br) can be attached to different carbon atoms in the carbon chain, resulting in different positional isomers.
1. 1-Bromobutane: In this isomer, the bromine atom is attached to the first carbon atom in the butane chain.
2. 2-Bromobutane: Here, the bromine atom is attached to the second carbon atom in the butane chain.
3. 3-Bromobutane: In this isomer, the bromine atom is attached to the third carbon atom in the butane chain.
4. 4-Bromobutane: In this isomer, the bromine atom is attached to the fourth carbon atom in the butane chain.
Explanation:
Positional isomerism arises due to the different arrangements of substituents or functional groups on a carbon chain. The position of attachment of these groups can greatly influence the physical and chemical properties of a compound.
Key Points:
- Positional isomers have the same molecular formula but different structural arrangements.
- The position of functional groups or substituents on the carbon chain determines the positional isomerism.
- Positional isomers exhibit different physical and chemical properties due to variations in the arrangement of atoms.
- The systematic naming of positional isomers is based on the numbering of the carbon atoms in the chain.
- Positional isomerism is commonly observed in organic compounds with multiple carbon atoms and functional groups.
Conclusion:
Positional isomerism is a type of structural isomerism where compounds have the same molecular formula but differ in the position of functional groups or substituents on the carbon skeleton. Understanding positional isomerism is important in organic chemistry as it helps in predicting and explaining the different properties and behaviors of compounds with similar molecular formulas.
Example:
Let's consider the compound C4H9Br, which can exhibit positional isomerism. In this case, the bromine atom (Br) can be attached to different carbon atoms in the carbon chain, resulting in different positional isomers.
1. 1-Bromobutane: In this isomer, the bromine atom is attached to the first carbon atom in the butane chain.
2. 2-Bromobutane: Here, the bromine atom is attached to the second carbon atom in the butane chain.
3. 3-Bromobutane: In this isomer, the bromine atom is attached to the third carbon atom in the butane chain.
4. 4-Bromobutane: In this isomer, the bromine atom is attached to the fourth carbon atom in the butane chain.
Explanation:
Positional isomerism arises due to the different arrangements of substituents or functional groups on a carbon chain. The position of attachment of these groups can greatly influence the physical and chemical properties of a compound.
Key Points:
- Positional isomers have the same molecular formula but different structural arrangements.
- The position of functional groups or substituents on the carbon chain determines the positional isomerism.
- Positional isomers exhibit different physical and chemical properties due to variations in the arrangement of atoms.
- The systematic naming of positional isomers is based on the numbering of the carbon atoms in the chain.
- Positional isomerism is commonly observed in organic compounds with multiple carbon atoms and functional groups.
Conclusion:
Positional isomerism is a type of structural isomerism where compounds have the same molecular formula but differ in the position of functional groups or substituents on the carbon skeleton. Understanding positional isomerism is important in organic chemistry as it helps in predicting and explaining the different properties and behaviors of compounds with similar molecular formulas.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
what is ment by positional isomerism please explain with example
Question Description
what is ment by positional isomerism please explain with example for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about what is ment by positional isomerism please explain with example covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for what is ment by positional isomerism please explain with example.
what is ment by positional isomerism please explain with example for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about what is ment by positional isomerism please explain with example covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for what is ment by positional isomerism please explain with example.
Solutions for what is ment by positional isomerism please explain with example in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of what is ment by positional isomerism please explain with example defined & explained in the simplest way possible. Besides giving the explanation of
what is ment by positional isomerism please explain with example, a detailed solution for what is ment by positional isomerism please explain with example has been provided alongside types of what is ment by positional isomerism please explain with example theory, EduRev gives you an
ample number of questions to practice what is ment by positional isomerism please explain with example tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















