Class 9 Exam > Class 9 Questions > How does electron distributed in different or...
Start Learning for Free
How does electron distributed in different orbits?
Verified Answer
How does electron distributed in different orbits?
The shells begin from the centre and gradually move outwards. So K shell will always have minimum energy. Similarly, L shell is a little away from nucleus so it will have higher energy than K shell. The outermost shell will have maximum energy. Now it is important to understand the distribution and arrangement of electrons in the atoms of any elements in the different energy levels.
An atom of any element is most stable when it has minimum energy. An atom will first fill the lowest energy level so as to attain the state of minimum energy. Gradually, the electrons will fill the higher energy levels. Therefore, electrons will first fill K shell, then L shell, M shell, N shell, and so on.
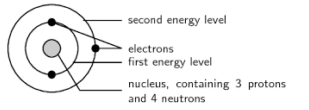
 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
How does electron distributed in different orbits?
Electron Distribution in Different Orbits
Introduction:
Electron distribution refers to the arrangement of electrons in different energy levels or orbits around the nucleus of an atom. The distribution of electrons follows certain rules and principles, such as the Aufbau principle, Pauli exclusion principle, and Hund's rule. These principles govern the filling of electron orbitals in a specific order.
1. Electron Orbitals:
Electron orbitals are regions of space around the nucleus where electrons are most likely to be found. These orbitals have different shapes and energy levels. The four types of orbitals are s, p, d, and f, each with a specific shape and capacity to hold a certain number of electrons.
2. Aufbau Principle:
The Aufbau principle states that electrons fill the lowest energy orbitals first before moving to higher energy orbitals. This means that electrons will occupy the 1s orbital before moving to the 2s orbital, and so on. The order of filling orbitals is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, and so on.
3. Pauli Exclusion Principle:
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons with opposite spins. For example, the 1s orbital can hold two electrons with opposite spins, one with spin up and the other with spin down.
4. Hund's Rule:
Hund's rule states that when filling orbitals of equal energy (degenerate orbitals), electrons will occupy separate orbitals with parallel spins before pairing up. This is known as the "one-electron-per-orbital" rule. It ensures that electrons are distributed in a way that maximizes their total spin.
5. Electron Distribution Examples:
- For example, consider the electron distribution of carbon (atomic number 6). The first two electrons fill the 1s orbital, the next two fill the 2s orbital, and the remaining two fill the 2p orbital. Thus, the electron configuration of carbon is 1s2 2s2 2p2.
- Similarly, the electron distribution of oxygen (atomic number 8) is 1s2 2s2 2p4. The first two electrons fill the 1s orbital, the next two fill the 2s orbital, and the remaining four fill the 2p orbital.
- The electron distribution of sodium (atomic number 11) is 1s2 2s2 2p6 3s1. The first two electrons fill the 1s orbital, the next two fill the 2s orbital, the next six fill the 2p orbital, and the last electron occupies the 3s orbital.
Conclusion:
In summary, electron distribution in different orbits follows the Aufbau principle, Pauli exclusion principle, and Hund's rule. These principles determine the order of filling orbitals and the maximum number of electrons each orbital can hold. Understanding electron distribution is essential in understanding the chemical and physical properties of elements.
Introduction:
Electron distribution refers to the arrangement of electrons in different energy levels or orbits around the nucleus of an atom. The distribution of electrons follows certain rules and principles, such as the Aufbau principle, Pauli exclusion principle, and Hund's rule. These principles govern the filling of electron orbitals in a specific order.
1. Electron Orbitals:
Electron orbitals are regions of space around the nucleus where electrons are most likely to be found. These orbitals have different shapes and energy levels. The four types of orbitals are s, p, d, and f, each with a specific shape and capacity to hold a certain number of electrons.
2. Aufbau Principle:
The Aufbau principle states that electrons fill the lowest energy orbitals first before moving to higher energy orbitals. This means that electrons will occupy the 1s orbital before moving to the 2s orbital, and so on. The order of filling orbitals is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, and so on.
3. Pauli Exclusion Principle:
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons with opposite spins. For example, the 1s orbital can hold two electrons with opposite spins, one with spin up and the other with spin down.
4. Hund's Rule:
Hund's rule states that when filling orbitals of equal energy (degenerate orbitals), electrons will occupy separate orbitals with parallel spins before pairing up. This is known as the "one-electron-per-orbital" rule. It ensures that electrons are distributed in a way that maximizes their total spin.
5. Electron Distribution Examples:
- For example, consider the electron distribution of carbon (atomic number 6). The first two electrons fill the 1s orbital, the next two fill the 2s orbital, and the remaining two fill the 2p orbital. Thus, the electron configuration of carbon is 1s2 2s2 2p2.
- Similarly, the electron distribution of oxygen (atomic number 8) is 1s2 2s2 2p4. The first two electrons fill the 1s orbital, the next two fill the 2s orbital, and the remaining four fill the 2p orbital.
- The electron distribution of sodium (atomic number 11) is 1s2 2s2 2p6 3s1. The first two electrons fill the 1s orbital, the next two fill the 2s orbital, the next six fill the 2p orbital, and the last electron occupies the 3s orbital.
Conclusion:
In summary, electron distribution in different orbits follows the Aufbau principle, Pauli exclusion principle, and Hund's rule. These principles determine the order of filling orbitals and the maximum number of electrons each orbital can hold. Understanding electron distribution is essential in understanding the chemical and physical properties of elements.
Community Answer
How does electron distributed in different orbits?
Electron distribution is based on the rule of 2n^2
n is number is shells
n is number is shells
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
How does electron distributed in different orbits?
Question Description
How does electron distributed in different orbits? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about How does electron distributed in different orbits? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How does electron distributed in different orbits?.
How does electron distributed in different orbits? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about How does electron distributed in different orbits? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How does electron distributed in different orbits?.
Solutions for How does electron distributed in different orbits? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of How does electron distributed in different orbits? defined & explained in the simplest way possible. Besides giving the explanation of
How does electron distributed in different orbits?, a detailed solution for How does electron distributed in different orbits? has been provided alongside types of How does electron distributed in different orbits? theory, EduRev gives you an
ample number of questions to practice How does electron distributed in different orbits? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























