Class 11 Exam > Class 11 Questions > EWho discovered protons, electrons and neutro...
Start Learning for Free
EWho discovered protons, electrons and neutrons
? Related: History of Atoms
Verified Answer
EWho discovered protons, electrons and neutrons Related: History of A...
Neutron-The neutron was discovered in 1932 by the English physicist James Chadwick.
In 1920, Ernest Rutherford postulated that there were neutral, massive particles in the nucleus of atoms. This conclusion arose from the disparity between an element's atomic number (protons = electrons) and its atomic mass (usually in excess of the mass of the known protons present). James Chadwick was assigned the task of tracking down evidence of Rutherford's tightly bound "proton-electron pair" or neutron.
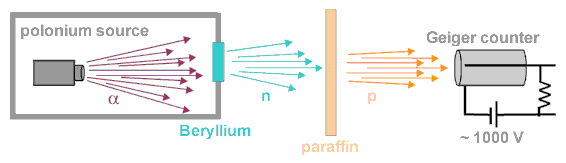
In 1930 it was discovered that Beryllium, when bombarded by alpha particles, emitted a very energetic stream of radiation. This stream was originally thought to be gamma radiation. However, further investigations into the properties of the radiation revealed contradictory results. Like gamma rays, these rays were extremely penetrating and since they were not deflected upon passing through a magnetic field, neutral. However, unlike gamma rays, these rays did not discharge charged electroscopes (the photoelectric effect). Irene Curie and her husband discovered that when a beam of this radiation hit a substance rich in protons, for example paraffin, protons were knocked loose which could be easily detected by a Geiger counter.
In 1932, Chadwick proposed that this particle was Rutherford's neutron.
Electron- J.J Thomson
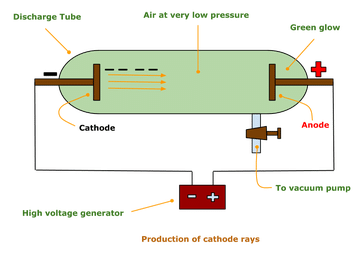
Electron was discovered by J. J. Thomson in 1897 when he was studying the properties of cathode ray.J. J. Thomson constructed a glass tube which was partially evacuated i.e. much of the air was pumped out of the tube. Then he applied a high electrical voltage between two electrodes at either end of the tube. He detected that a stream of particle (ray) was coming out from the negatively charged electrode (cathode) to positively charged electrode (anode). This ray is called cathode ray and the whole construction is called cathode ray tube. The schematic of a cathode ray tube is given.
Proton- Goldstein
Since the atom as a whole is electrically neutral and the presence of negatively charged particles in it was established ,therefore, it was thought that some positively charged particles must also be present in the atom.
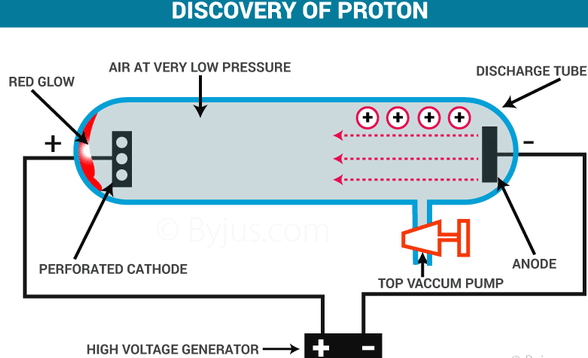
Goldsstein in 1886 performed discharge tube experiment in which he took perforated cathode and a gas at low pressure was kept inside the tube, as before.
On passing high voltage between the electrodes ,it was found that some rays were coming from the side of the anode which passed through the holes in the cathode and produced green fluroscense on the opposite glass wall coated with zinc sulphide. These rays were called anode rays or canal rays or positive rays.
Properties of anode rays.
1) They travel in straight line.
2) They are made up of material particles.
3) They are positively charged.
4) The value of e/m depend upon the nature of gas taken inside the discharge tube.
5) The value of the charge on the particles constituent the anode rays is also found to depend upon the nature of the gas taken inside the discharge tube.
6) The mass of the particle constituting the anode rays is also found to be different for different gases taken in discharge tube.
The charge on these particles is found to be same as that on the electron i.e. 1.6 * 10^-19 coulombs per gram.
The ratio, charge/mass, for each of the particle is found to be 9.58 * 10^-24 g.
These particles were termed as protons.
 This question is part of UPSC exam. View all Class 11 courses
This question is part of UPSC exam. View all Class 11 courses
Most Upvoted Answer
EWho discovered protons, electrons and neutrons Related: History of A...
Discovery of Protons, Electrons, and Neutrons
Protons, electrons, and neutrons are fundamental particles that make up atoms. Each of these particles was discovered by different scientists through various experiments.
Protons
- Protons were discovered by Ernest Rutherford in 1919 through his gold foil experiment. He observed that some alpha particles were deflected at large angles, indicating the presence of a dense, positively charged nucleus within an atom. This led to the discovery of protons, which are positively charged particles located in the nucleus of an atom.
Electrons
- Electrons were discovered by J.J. Thomson in 1897 through his cathode ray tube experiment. He observed that cathode rays were deflected by electric and magnetic fields, leading him to conclude that they were negatively charged particles. These particles were later named electrons and are located outside the nucleus of an atom.
Neutrons
- Neutrons were discovered by James Chadwick in 1932. He conducted experiments bombarding beryllium with alpha particles, which resulted in the emission of a previously unknown neutral particle. This particle was identified as the neutron, which is a neutral particle located in the nucleus of an atom.
These discoveries revolutionized our understanding of the structure of atoms and laid the foundation for modern atomic theory. Together, protons, electrons, and neutrons form the building blocks of matter and play essential roles in determining the properties of different elements.
Protons, electrons, and neutrons are fundamental particles that make up atoms. Each of these particles was discovered by different scientists through various experiments.
Protons
- Protons were discovered by Ernest Rutherford in 1919 through his gold foil experiment. He observed that some alpha particles were deflected at large angles, indicating the presence of a dense, positively charged nucleus within an atom. This led to the discovery of protons, which are positively charged particles located in the nucleus of an atom.
Electrons
- Electrons were discovered by J.J. Thomson in 1897 through his cathode ray tube experiment. He observed that cathode rays were deflected by electric and magnetic fields, leading him to conclude that they were negatively charged particles. These particles were later named electrons and are located outside the nucleus of an atom.
Neutrons
- Neutrons were discovered by James Chadwick in 1932. He conducted experiments bombarding beryllium with alpha particles, which resulted in the emission of a previously unknown neutral particle. This particle was identified as the neutron, which is a neutral particle located in the nucleus of an atom.
These discoveries revolutionized our understanding of the structure of atoms and laid the foundation for modern atomic theory. Together, protons, electrons, and neutrons form the building blocks of matter and play essential roles in determining the properties of different elements.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
EWho discovered protons, electrons and neutrons Related: History of Atoms?
Question Description
EWho discovered protons, electrons and neutrons Related: History of Atoms? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about EWho discovered protons, electrons and neutrons Related: History of Atoms? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for EWho discovered protons, electrons and neutrons Related: History of Atoms?.
EWho discovered protons, electrons and neutrons Related: History of Atoms? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about EWho discovered protons, electrons and neutrons Related: History of Atoms? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for EWho discovered protons, electrons and neutrons Related: History of Atoms?.
Solutions for EWho discovered protons, electrons and neutrons Related: History of Atoms? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of EWho discovered protons, electrons and neutrons Related: History of Atoms? defined & explained in the simplest way possible. Besides giving the explanation of
EWho discovered protons, electrons and neutrons Related: History of Atoms?, a detailed solution for EWho discovered protons, electrons and neutrons Related: History of Atoms? has been provided alongside types of EWho discovered protons, electrons and neutrons Related: History of Atoms? theory, EduRev gives you an
ample number of questions to practice EWho discovered protons, electrons and neutrons Related: History of Atoms? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























