Class 9 Exam > Class 9 Questions > Difference between elements and compounds.?
Start Learning for Free
Difference between elements and compounds.?
Most Upvoted Answer
Difference between elements and compounds.?
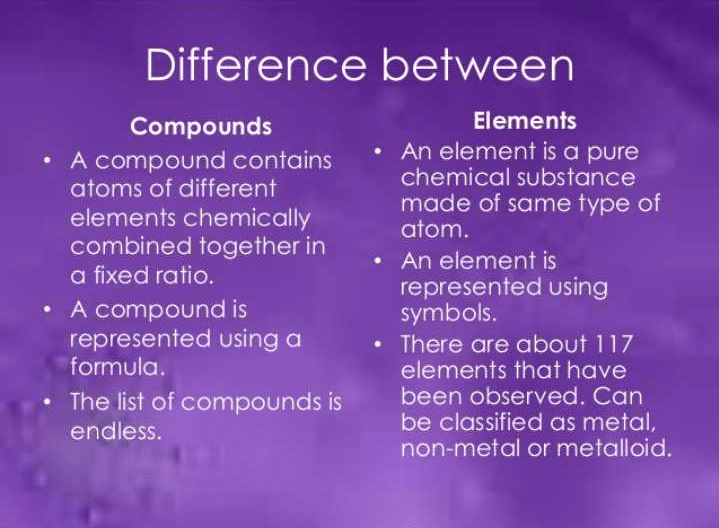
Community Answer
Difference between elements and compounds.?
Introduction:
Elements and compounds are both types of substances that make up the matter around us. While they are similar in some ways, they also have distinct differences. In this explanation, we will delve into the details of elements and compounds, highlighting their characteristics and contrasting features.
Elements:
An element is a pure substance that consists of only one type of atom. Atoms are the basic building blocks of matter, and each element is defined by the number of protons in its atomic nucleus. There are 118 known elements, ranging from hydrogen (the lightest element) to oganesson (the heaviest element). Some common examples of elements include oxygen, carbon, gold, and helium.
Characteristics of Elements:
Elements possess several key characteristics:
1. Atomic Structure: Elements are composed of atoms with the same number of protons. For example, all carbon atoms have six protons.
2. Chemical Symbol: Each element is represented by a chemical symbol, such as H for hydrogen and O for oxygen.
3. Physical and Chemical Properties: Elements have unique physical and chemical properties, which define their behavior and reactivity. For instance, oxygen is a gas at room temperature, while gold is a solid metal.
4. Periodic Table: Elements are organized in the periodic table based on their atomic number, electron configuration, and recurring chemical properties.
Compounds:
A compound is a substance formed when two or more elements chemically combine in a fixed ratio. The elements in a compound are held together by chemical bonds, resulting in a new substance with properties distinct from its constituent elements. For example, water (H2O) is a compound composed of two hydrogen atoms and one oxygen atom.
Characteristics of Compounds:
Compounds possess several key characteristics:
1. Chemical Formula: Compounds are represented by a chemical formula that indicates the type and number of atoms present. For instance, the formula for carbon dioxide (CO2) indicates one carbon atom and two oxygen atoms.
2. Fixed Composition: Compounds have a fixed composition, meaning they always contain the same elements in the same ratio. For example, water is always composed of two hydrogen atoms and one oxygen atom.
3. Unique Properties: Compounds exhibit properties that are different from those of their constituent elements. For instance, sodium (a highly reactive metal) and chlorine (a poisonous gas) individually have distinct properties, but when combined, they form sodium chloride (table salt), which is a stable compound.
Key Differences:
1. Composition: Elements are composed of a single type of atom, while compounds are composed of two or more different types of atoms.
2. Chemical Bonds: Elements do not involve chemical bonding, whereas compounds are held together by chemical bonds.
3. Ratio: Elements do not have a fixed ratio, while compounds have a fixed ratio of atoms.
4. Properties: Elements possess unique properties, whereas compounds have properties distinct from their constituent elements.
Conclusion:
In summary, elements consist of single types of atoms, while compounds are formed by the combination of different elements in fixed ratios. Elements
Elements and compounds are both types of substances that make up the matter around us. While they are similar in some ways, they also have distinct differences. In this explanation, we will delve into the details of elements and compounds, highlighting their characteristics and contrasting features.
Elements:
An element is a pure substance that consists of only one type of atom. Atoms are the basic building blocks of matter, and each element is defined by the number of protons in its atomic nucleus. There are 118 known elements, ranging from hydrogen (the lightest element) to oganesson (the heaviest element). Some common examples of elements include oxygen, carbon, gold, and helium.
Characteristics of Elements:
Elements possess several key characteristics:
1. Atomic Structure: Elements are composed of atoms with the same number of protons. For example, all carbon atoms have six protons.
2. Chemical Symbol: Each element is represented by a chemical symbol, such as H for hydrogen and O for oxygen.
3. Physical and Chemical Properties: Elements have unique physical and chemical properties, which define their behavior and reactivity. For instance, oxygen is a gas at room temperature, while gold is a solid metal.
4. Periodic Table: Elements are organized in the periodic table based on their atomic number, electron configuration, and recurring chemical properties.
Compounds:
A compound is a substance formed when two or more elements chemically combine in a fixed ratio. The elements in a compound are held together by chemical bonds, resulting in a new substance with properties distinct from its constituent elements. For example, water (H2O) is a compound composed of two hydrogen atoms and one oxygen atom.
Characteristics of Compounds:
Compounds possess several key characteristics:
1. Chemical Formula: Compounds are represented by a chemical formula that indicates the type and number of atoms present. For instance, the formula for carbon dioxide (CO2) indicates one carbon atom and two oxygen atoms.
2. Fixed Composition: Compounds have a fixed composition, meaning they always contain the same elements in the same ratio. For example, water is always composed of two hydrogen atoms and one oxygen atom.
3. Unique Properties: Compounds exhibit properties that are different from those of their constituent elements. For instance, sodium (a highly reactive metal) and chlorine (a poisonous gas) individually have distinct properties, but when combined, they form sodium chloride (table salt), which is a stable compound.
Key Differences:
1. Composition: Elements are composed of a single type of atom, while compounds are composed of two or more different types of atoms.
2. Chemical Bonds: Elements do not involve chemical bonding, whereas compounds are held together by chemical bonds.
3. Ratio: Elements do not have a fixed ratio, while compounds have a fixed ratio of atoms.
4. Properties: Elements possess unique properties, whereas compounds have properties distinct from their constituent elements.
Conclusion:
In summary, elements consist of single types of atoms, while compounds are formed by the combination of different elements in fixed ratios. Elements
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
Difference between elements and compounds.?
Question Description
Difference between elements and compounds.? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Difference between elements and compounds.? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between elements and compounds.?.
Difference between elements and compounds.? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Difference between elements and compounds.? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between elements and compounds.?.
Solutions for Difference between elements and compounds.? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of Difference between elements and compounds.? defined & explained in the simplest way possible. Besides giving the explanation of
Difference between elements and compounds.?, a detailed solution for Difference between elements and compounds.? has been provided alongside types of Difference between elements and compounds.? theory, EduRev gives you an
ample number of questions to practice Difference between elements and compounds.? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























