JEE Exam > JEE Questions > Paragraph for Questions 11 and 12The β-d...
Start Learning for Free
Paragraph for Questions 11 and 12
The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory, a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore, considering the decay of a neutron as a two-body decay process, it was predicted theoretically that the kinetic energy of the electron should be a constant. But experimentally, it was observed that the electron kinetic energy has continuous spectrum. Considering a three-body decay process, i.e. 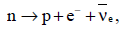
around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino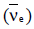 to be massless and possessing negligible energy, and the neutron to be at rest, momentum and energy conservation principles are applied. From this calculation, the maximum kinetic energy of the electron is 0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.
to be massless and possessing negligible energy, and the neutron to be at rest, momentum and energy conservation principles are applied. From this calculation, the maximum kinetic energy of the electron is 0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.
around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino
Q. If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, what
should be the range of the kinetic energy, K, of the electron?
should be the range of the kinetic energy, K, of the electron?
- a)0 < K < 0.8 x 106 eV
- b)3.0 eV < K < 0.8 x 106 eV
- c)3.0eV < K < 0.8 x 106 eV
- d)0 < K < 0.8 x 106 eV
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |

|
Explore Courses for JEE exam
|

|
Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer?
Question Description
Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer?.
Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Paragraph for Questions 11 and 12The β-decay process, discovered around 1900, is basically the decay of a neutron (n). In the laboratory,a proton (p) and an electron (e–) are observed as the decay products of the neutron. Therefore,considering the decay of a neutron as a two-body decay process, it was predicted theoretically that thekinetic energy of the electron should be a constant. But experimentally, it was observed that the electronkinetic energy has continuous spectrum. Considering a three-body decay process, i.e. around 1930, Pauli explained the observed electron energy spectrum. Assuming the anti-neutrino tobe massless and possessing negligible energy, and the neutron to be at rest, momentum and energyconservation principles are applied. From this calculation, the maximum kinetic energy of the electron is0.8 × 106 eV. The kinetic energy carried by the proton is only the recoil energy.Q.If the anti-neutrino had a mass of 3eV/c2 (where c is the speed of light) instead of zero mass, whatshould be the range of the kinetic energy, K, of the electron?a)0 < K <0.8 x 106 eVb)3.0 eV < K <0.8 x 106 eVc)3.0eV < K <0.8 x 106 eVd)0 < K < 0.8 x 106 eVCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















