Class 12 Exam > Class 12 Questions > Monomer of Nylon - 6:a)Caprolactumb)Caprolact...
Start Learning for Free
Monomer of Nylon - 6:
- a)Caprolactum
- b)Caprolactone
- c)Acrylonitrile
- d)Isoprene
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)Isopr...
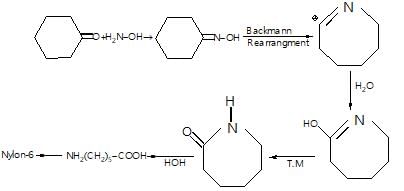
Monomer → Caprolactum
Most Upvoted Answer
Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)Isopr...
Monomer of Nylon-6: Caprolactam
Caprolactam is the monomer of Nylon-6. It is a cyclic amide, which means that it contains a carbonyl group and an amino group in its structure. Caprolactam is synthesized from cyclohexanone and ammonium sulfate.
Polymerization of Caprolactam
The polymerization of caprolactam involves the opening of the lactam ring and the formation of a long chain of repeating units. This process is called ring-opening polymerization.
The steps involved in the polymerization of caprolactam are:
1. Initiation: A catalyst is added to caprolactam to initiate the polymerization process. The most common catalyst is anionic polymerization.
2. Propagation: The lactam ring opens, and the carbonyl group of one molecule reacts with the amino group of another molecule to form a peptide bond. This process is repeated to form a long chain of repeating units.
3. Termination: The polymerization process stops when all of the caprolactam monomers have been consumed.
Properties of Nylon-6
Nylon-6 is a thermoplastic material that is characterized by its high strength, toughness, and abrasion resistance. Nylon-6 is also resistant to chemicals, heat, and UV radiation.
Applications of Nylon-6
Nylon-6 is used in a wide range of applications, including:
1. Textiles: Nylon-6 is used to make clothing, such as stockings and swimsuits, as well as carpet fibers and upholstery.
2. Engineering plastics: Nylon-6 is used to make a variety of engineering plastics, including gears, bearings, and connectors.
3. Films: Nylon-6 is used to make films for packaging and agricultural applications.
Conclusion
Caprolactam is the monomer of Nylon-6, and its polymerization involves the opening of the lactam ring and the formation of a long chain of repeating units. Nylon-6 is a thermoplastic material that is used in a wide range of applications, including textiles, engineering plastics, and films.
Caprolactam is the monomer of Nylon-6. It is a cyclic amide, which means that it contains a carbonyl group and an amino group in its structure. Caprolactam is synthesized from cyclohexanone and ammonium sulfate.
Polymerization of Caprolactam
The polymerization of caprolactam involves the opening of the lactam ring and the formation of a long chain of repeating units. This process is called ring-opening polymerization.
The steps involved in the polymerization of caprolactam are:
1. Initiation: A catalyst is added to caprolactam to initiate the polymerization process. The most common catalyst is anionic polymerization.
2. Propagation: The lactam ring opens, and the carbonyl group of one molecule reacts with the amino group of another molecule to form a peptide bond. This process is repeated to form a long chain of repeating units.
3. Termination: The polymerization process stops when all of the caprolactam monomers have been consumed.
Properties of Nylon-6
Nylon-6 is a thermoplastic material that is characterized by its high strength, toughness, and abrasion resistance. Nylon-6 is also resistant to chemicals, heat, and UV radiation.
Applications of Nylon-6
Nylon-6 is used in a wide range of applications, including:
1. Textiles: Nylon-6 is used to make clothing, such as stockings and swimsuits, as well as carpet fibers and upholstery.
2. Engineering plastics: Nylon-6 is used to make a variety of engineering plastics, including gears, bearings, and connectors.
3. Films: Nylon-6 is used to make films for packaging and agricultural applications.
Conclusion
Caprolactam is the monomer of Nylon-6, and its polymerization involves the opening of the lactam ring and the formation of a long chain of repeating units. Nylon-6 is a thermoplastic material that is used in a wide range of applications, including textiles, engineering plastics, and films.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer?
Question Description
Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer?.
Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Monomer of Nylon - 6:a)Caprolactumb)Caprolactonec)Acrylonitriled)IsopreneCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















