Class 11 Exam > Class 11 Questions > Which one of the following compounds is resis...
Start Learning for Free
Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?
- a)Methyl acetate
- b)Acetonitrile [1998]
- c)Diethyl ether
- d)Acetamide
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which one of the following compounds is resistant to nucleophilic atta...
The compound is diethyl ether (CH3CH2)2O which is resistant to nucleophilic attack by hydroxyl ion due to absent of double or triple bond, whereas all other compounds given are unsaturated
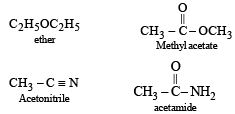
Most Upvoted Answer
Which one of the following compounds is resistant to nucleophilic atta...
Resistant to nucleophilic attack by hydroxyl ions: Diethyl ether
Nucleophilic attack is a chemical reaction in which an electron-rich nucleophile attacks an electron-deficient electrophile, resulting in the formation of a new chemical bond. Hydroxyl ions (OH-) are a common nucleophile.
Diethyl ether is resistant to nucleophilic attack by hydroxyl ions due to the following reasons:
- Lack of a polar functional group: Diethyl ether is a simple ether, which lacks a polar functional group such as carbonyl or carboxyl that can easily undergo nucleophilic attack. This makes it less susceptible to nucleophilic attack by hydroxyl ions.
- Weak basicity: Diethyl ether is a weakly basic molecule, which means it has a low tendency to attract protons or accept electrons. This makes it less likely to react with hydroxyl ions, which are strong bases.
- Steric hindrance: The ethyl groups in diethyl ether create steric hindrance, which means they hinder the approach of nucleophiles such as hydroxyl ions. This makes it more difficult for hydroxyl ions to attack diethyl ether.
In contrast, methyl acetate, acetamide, and acetonitrile all contain polar functional groups that can easily undergo nucleophilic attack by hydroxyl ions. Therefore, they are more susceptible to nucleophilic attack than diethyl ether.
Overall, diethyl ether is a relatively inert compound that is resistant to nucleophilic attack by hydroxyl ions, making it a useful solvent in many chemical reactions.
Nucleophilic attack is a chemical reaction in which an electron-rich nucleophile attacks an electron-deficient electrophile, resulting in the formation of a new chemical bond. Hydroxyl ions (OH-) are a common nucleophile.
Diethyl ether is resistant to nucleophilic attack by hydroxyl ions due to the following reasons:
- Lack of a polar functional group: Diethyl ether is a simple ether, which lacks a polar functional group such as carbonyl or carboxyl that can easily undergo nucleophilic attack. This makes it less susceptible to nucleophilic attack by hydroxyl ions.
- Weak basicity: Diethyl ether is a weakly basic molecule, which means it has a low tendency to attract protons or accept electrons. This makes it less likely to react with hydroxyl ions, which are strong bases.
- Steric hindrance: The ethyl groups in diethyl ether create steric hindrance, which means they hinder the approach of nucleophiles such as hydroxyl ions. This makes it more difficult for hydroxyl ions to attack diethyl ether.
In contrast, methyl acetate, acetamide, and acetonitrile all contain polar functional groups that can easily undergo nucleophilic attack by hydroxyl ions. Therefore, they are more susceptible to nucleophilic attack than diethyl ether.
Overall, diethyl ether is a relatively inert compound that is resistant to nucleophilic attack by hydroxyl ions, making it a useful solvent in many chemical reactions.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer?.
Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?a)Methyl acetateb)Acetonitrile [1998]c)Diethyl etherd)AcetamideCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























