Class 11 Exam > Class 11 Questions > Water gas is produced by [1992]a)Passing stea...
Start Learning for Free
Water gas is produced by [1992]
- a)Passing steam through a red hot coke bed
- b)Saturating hydrogen with moisture
- c)Mixing oxygen and hydrogen in the ratio of 1 : 2
- d)Heating a mixture of CO2 and CH4 in petroleum refineries.
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Water gas is produced by [1992]a)Passing steam through a red hot coke ...
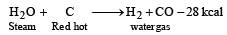
Water gas is made by blowing steam through the layer of incandescent coal.
Most Upvoted Answer
Water gas is produced by [1992]a)Passing steam through a red hot coke ...
Answer:
Water gas, also known as synthesis gas or syngas, is a mixture of carbon monoxide (CO) and hydrogen gas (H2). It is commonly used as a fuel and as a starting material for the production of various chemicals. In the given options, the correct method for the production of water gas is passing steam through a red hot coke bed.
Explanation:
Water gas is produced by a process called steam reforming, which involves the reaction of steam with a carbonaceous material, such as coke. This process can be explained in the following steps:
1. Preparation of coke: Coke is a carbonaceous material that is obtained by heating coal in the absence of air. It is a porous substance that provides a large surface area for the reaction to occur.
2. Heating the coke: The coke is then heated to a high temperature, typically around 1000-1100°C, in a reaction vessel or furnace. This is done in the absence of oxygen to prevent the complete combustion of the coke.
3. Passing steam through the coke bed: Steam, which is water in the gaseous state, is then passed through the red-hot coke bed. The high temperature of the coke promotes the reaction between steam and carbon to produce carbon monoxide and hydrogen gas.
The overall reaction can be represented as follows:
C(s) + H2O(g) → CO(g) + H2(g)
In this reaction, carbon from the coke reacts with steam to produce carbon monoxide and hydrogen gas. These gases can be separated and used for various purposes, such as fuel for heating and as starting materials for the production of chemicals.
It is important to note that the other options listed in the question do not describe the correct method for the production of water gas. Saturating hydrogen with moisture does not involve the use of coke or the reaction with steam. Mixing oxygen and hydrogen in the ratio of 1:2 would result in the production of water (H2O) and not water gas. Heating a mixture of CO2 and CH4 in petroleum refineries does not lead to the production of water gas either.
Therefore, the correct answer is option 'A' - Passing steam through a red hot coke bed.
Water gas, also known as synthesis gas or syngas, is a mixture of carbon monoxide (CO) and hydrogen gas (H2). It is commonly used as a fuel and as a starting material for the production of various chemicals. In the given options, the correct method for the production of water gas is passing steam through a red hot coke bed.
Explanation:
Water gas is produced by a process called steam reforming, which involves the reaction of steam with a carbonaceous material, such as coke. This process can be explained in the following steps:
1. Preparation of coke: Coke is a carbonaceous material that is obtained by heating coal in the absence of air. It is a porous substance that provides a large surface area for the reaction to occur.
2. Heating the coke: The coke is then heated to a high temperature, typically around 1000-1100°C, in a reaction vessel or furnace. This is done in the absence of oxygen to prevent the complete combustion of the coke.
3. Passing steam through the coke bed: Steam, which is water in the gaseous state, is then passed through the red-hot coke bed. The high temperature of the coke promotes the reaction between steam and carbon to produce carbon monoxide and hydrogen gas.
The overall reaction can be represented as follows:
C(s) + H2O(g) → CO(g) + H2(g)
In this reaction, carbon from the coke reacts with steam to produce carbon monoxide and hydrogen gas. These gases can be separated and used for various purposes, such as fuel for heating and as starting materials for the production of chemicals.
It is important to note that the other options listed in the question do not describe the correct method for the production of water gas. Saturating hydrogen with moisture does not involve the use of coke or the reaction with steam. Mixing oxygen and hydrogen in the ratio of 1:2 would result in the production of water (H2O) and not water gas. Heating a mixture of CO2 and CH4 in petroleum refineries does not lead to the production of water gas either.
Therefore, the correct answer is option 'A' - Passing steam through a red hot coke bed.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer?
Question Description
Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer?.
Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer?.
Solutions for Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Water gas is produced by [1992]a)Passing steam through a red hot coke bedb)Saturating hydrogen with moisturec)Mixing oxygen and hydrogen in the ratio of 1 : 2d)Heating a mixture of CO2 and CH4 in petroleum refineries.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























