Class 12 Exam > Class 12 Questions > Chloropicrin is obtained by the reaction of [...
Start Learning for Free
Chloropicrin is obtained by the reaction of [2004]
- a)steam on carbon tetrachloride
- b)nitric acid on chlorobenzene
- c)chlorine on picric acid
- d)nitric acid on chloroform
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Chloropicrin is obtained by the reaction of [2004]a)steam on carbon te...
Chloropicrin is nitrochloroform. It is obtained by the nitration of chloroform with HNO3.
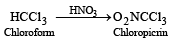
Chloropicrin is a liquids, poisonous and used as an insecticide and a war gas
Most Upvoted Answer
Chloropicrin is obtained by the reaction of [2004]a)steam on carbon te...
Nitric acid is used to obtain chloropicrin (trichloronitromethane) by reacting it with chloroform. This reaction is represented by the following chemical equation:
CHCl3 + HNO3 → CCl3NO2 + H2O
Explanation:
Chloropicrin, also known as trichloronitromethane (CCl3NO2), is a chemical compound used as a pesticide, fumigant, and tear gas. It is a yellowish liquid with a pungent odor.
The reaction between chloroform (CHCl3) and nitric acid (HNO3) is an example of a substitution reaction. In this reaction, one of the chlorine atoms in chloroform is replaced by a nitro group (NO2) from nitric acid.
Let's look at the reaction step by step:
1. Formation of chloroform:
Chloroform is a compound formed by the reaction of chlorine (Cl2) with methane (CH4). This reaction occurs in the presence of a catalyst, usually sunlight or heat.
CH4 + 3Cl2 → CHCl3 + 3HCl
2. Reaction with nitric acid:
When chloroform reacts with nitric acid, one of the chlorine atoms in chloroform is replaced by a nitro group (NO2) from nitric acid. This substitution reaction occurs due to the high reactivity of nitric acid.
CHCl3 + HNO3 → CCl3NO2 + H2O
The byproduct of this reaction is water (H2O). The product, chloropicrin (CCl3NO2), is formed as a result of the substitution of chlorine with a nitro group. Chloropicrin is a toxic compound and is commonly used as a pesticide and fumigant.
In conclusion, chloropicrin is obtained by the reaction of chloroform with nitric acid. This reaction involves the substitution of one of the chlorine atoms in chloroform with a nitro group from nitric acid.
CHCl3 + HNO3 → CCl3NO2 + H2O
Explanation:
Chloropicrin, also known as trichloronitromethane (CCl3NO2), is a chemical compound used as a pesticide, fumigant, and tear gas. It is a yellowish liquid with a pungent odor.
The reaction between chloroform (CHCl3) and nitric acid (HNO3) is an example of a substitution reaction. In this reaction, one of the chlorine atoms in chloroform is replaced by a nitro group (NO2) from nitric acid.
Let's look at the reaction step by step:
1. Formation of chloroform:
Chloroform is a compound formed by the reaction of chlorine (Cl2) with methane (CH4). This reaction occurs in the presence of a catalyst, usually sunlight or heat.
CH4 + 3Cl2 → CHCl3 + 3HCl
2. Reaction with nitric acid:
When chloroform reacts with nitric acid, one of the chlorine atoms in chloroform is replaced by a nitro group (NO2) from nitric acid. This substitution reaction occurs due to the high reactivity of nitric acid.
CHCl3 + HNO3 → CCl3NO2 + H2O
The byproduct of this reaction is water (H2O). The product, chloropicrin (CCl3NO2), is formed as a result of the substitution of chlorine with a nitro group. Chloropicrin is a toxic compound and is commonly used as a pesticide and fumigant.
In conclusion, chloropicrin is obtained by the reaction of chloroform with nitric acid. This reaction involves the substitution of one of the chlorine atoms in chloroform with a nitro group from nitric acid.

|
Explore Courses for Class 12 exam
|

|
Question Description
Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer?.
Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Chloropicrin is obtained by the reaction of [2004]a)steam on carbon tetrachlorideb)nitric acid on chlorobenzenec)chlorine on picric acidd)nitric acid on chloroformCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























