Class 11 Exam > Class 11 Questions > Which of the following is not a correct state...
Start Learning for Free
Which of the following is not a correct statement? [2006]
- a)The can on ical structures have no real existence
- b)Every AB5 molecule does in fact have square pyramidal structure
- c)Multiple bonds are always shorter than corresponding single bonds
- d)The electron-deficient molecules can act as Lewis acids
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which of the following is not a correct statement? [2006]a)The can on ...
Statement (a), (c), (d) are correct. Statement (b) is incorrect statement.
AB5 may have two structures as follows :
AB5 may have two structures as follows :
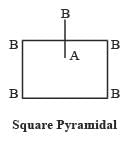
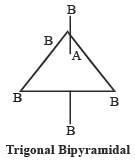
Most Upvoted Answer
Which of the following is not a correct statement? [2006]a)The can on ...
Explanation:
Option B is not a correct statement. It is false because not every AB5 molecule has a square pyramidal structure.
Let's understand the other options:
a) The canonical structures have no real existence:
- Canonical structures are hypothetical structures that represent the resonance hybrid of a molecule.
- They do not represent any actual physical structure of the molecule.
- Therefore, option A is a correct statement.
b) Every AB5 molecule does in fact have square pyramidal structure:
- AB5 molecules have 5 bonding pairs and no lone pairs of electrons.
- These molecules can have different shapes like trigonal bipyramidal, square pyramidal, or linear depending on the repulsion between the bonding pairs.
- Therefore, option B is a false statement.
c) Multiple bonds are always shorter than corresponding single bonds:
- Multiple bonds have more electrons shared between the atoms than single bonds.
- The increased electron density between the atoms pulls them closer to each other, making the bond shorter.
- Therefore, option C is a correct statement.
d) The electron-deficient molecules can act as Lewis acids:
- Lewis acids are electron acceptors, and they can accept a pair of electrons from a Lewis base.
- An electron-deficient molecule has a partially filled valence shell and can accept electrons to complete its octet.
- Therefore, option D is a correct statement.
In conclusion, the correct answer is option B.
Option B is not a correct statement. It is false because not every AB5 molecule has a square pyramidal structure.
Let's understand the other options:
a) The canonical structures have no real existence:
- Canonical structures are hypothetical structures that represent the resonance hybrid of a molecule.
- They do not represent any actual physical structure of the molecule.
- Therefore, option A is a correct statement.
b) Every AB5 molecule does in fact have square pyramidal structure:
- AB5 molecules have 5 bonding pairs and no lone pairs of electrons.
- These molecules can have different shapes like trigonal bipyramidal, square pyramidal, or linear depending on the repulsion between the bonding pairs.
- Therefore, option B is a false statement.
c) Multiple bonds are always shorter than corresponding single bonds:
- Multiple bonds have more electrons shared between the atoms than single bonds.
- The increased electron density between the atoms pulls them closer to each other, making the bond shorter.
- Therefore, option C is a correct statement.
d) The electron-deficient molecules can act as Lewis acids:
- Lewis acids are electron acceptors, and they can accept a pair of electrons from a Lewis base.
- An electron-deficient molecule has a partially filled valence shell and can accept electrons to complete its octet.
- Therefore, option D is a correct statement.
In conclusion, the correct answer is option B.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer?
Question Description
Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer?.
Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following is not a correct statement? [2006]a)The can on ical structures have no real existenceb)Every AB5 molecule does in fact have square pyramidal structurec)Multiple bonds are always shorter than corresponding single bondsd)The electron-deficient molecules can act as Lewis acidsCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.






















