Class 12 Exam > Class 12 Questions > Which of the following statements is wrong? a...
Start Learning for Free
Which of the following statements is wrong?
- a)An acidified solution of K2Cr2O7 liberates iodine from iodides
- b)In acidic solution, dichromate ions are converted to chromate ions
- c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3
- d)Potassium dichromate is used as a titrant for Fe2+ ions
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which of the following statements is wrong? a)An acidified solution of...
In acidic solution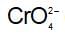 , changes to
, changes to 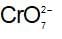
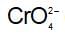 , changes to
, changes to 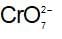
Most Upvoted Answer
Which of the following statements is wrong? a)An acidified solution of...
Statement b) In acidic solution, dichromate ions are converted to chromate ions
In acidic solution, dichromate ions (Cr2O7^2-) do not get converted to chromate ions (CrO4^2-). This statement is wrong.
Explanation:
1. Acidified solution of K2Cr2O7 liberates iodine from iodides:
- Potassium dichromate (K2Cr2O7) is a strong oxidizing agent. When it is added to an acidified solution containing iodide ions (I^-), it oxidizes iodide ions to iodine (I2).
- The reaction can be represented as: K2Cr2O7 + 6H+ + 6I^- → Cr^3+ + 3I2 + 2K+ + 3H2O
- The liberated iodine can be detected by its characteristic purple color.
2. Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3:
- Ammonium dichromate (NH4)2Cr2O7 is a complex compound that decomposes when heated. The decomposition reaction is highly exothermic, releasing heat and producing solid chromium(III) oxide (Cr2O3), nitrogen gas (N2), and water vapor (H2O).
- The reaction can be represented as: (NH4)2Cr2O7 → Cr2O3 + N2 + 4H2O
- This reaction is commonly known as the "volcano" or "ammonium dichromate" reaction due to the visual effect it produces, resembling a volcanic eruption.
3. Potassium dichromate is used as a titrant for Fe2+ ions:
- Potassium dichromate (K2Cr2O7) is commonly used as a primary standard in redox titrations.
- It is used as a titrant (a solution of known concentration) to determine the concentration of a reducing agent, such as Fe2+ ions (ferrous ions).
- In the titration, the orange-colored dichromate ions (Cr2O7^2-) are reduced to green-colored chromium(III) ions (Cr^3+), while the Fe2+ ions are oxidized to Fe3+ ions.
- The end point of the titration is usually indicated by a color change from orange to green, which signifies the complete reaction between the dichromate ions and the Fe2+ ions.
In summary, the correct statement is that potassium dichromate is used as a titrant for Fe2+ ions. The other statements are also true, except for statement b) which is wrong.
In acidic solution, dichromate ions (Cr2O7^2-) do not get converted to chromate ions (CrO4^2-). This statement is wrong.
Explanation:
1. Acidified solution of K2Cr2O7 liberates iodine from iodides:
- Potassium dichromate (K2Cr2O7) is a strong oxidizing agent. When it is added to an acidified solution containing iodide ions (I^-), it oxidizes iodide ions to iodine (I2).
- The reaction can be represented as: K2Cr2O7 + 6H+ + 6I^- → Cr^3+ + 3I2 + 2K+ + 3H2O
- The liberated iodine can be detected by its characteristic purple color.
2. Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3:
- Ammonium dichromate (NH4)2Cr2O7 is a complex compound that decomposes when heated. The decomposition reaction is highly exothermic, releasing heat and producing solid chromium(III) oxide (Cr2O3), nitrogen gas (N2), and water vapor (H2O).
- The reaction can be represented as: (NH4)2Cr2O7 → Cr2O3 + N2 + 4H2O
- This reaction is commonly known as the "volcano" or "ammonium dichromate" reaction due to the visual effect it produces, resembling a volcanic eruption.
3. Potassium dichromate is used as a titrant for Fe2+ ions:
- Potassium dichromate (K2Cr2O7) is commonly used as a primary standard in redox titrations.
- It is used as a titrant (a solution of known concentration) to determine the concentration of a reducing agent, such as Fe2+ ions (ferrous ions).
- In the titration, the orange-colored dichromate ions (Cr2O7^2-) are reduced to green-colored chromium(III) ions (Cr^3+), while the Fe2+ ions are oxidized to Fe3+ ions.
- The end point of the titration is usually indicated by a color change from orange to green, which signifies the complete reaction between the dichromate ions and the Fe2+ ions.
In summary, the correct statement is that potassium dichromate is used as a titrant for Fe2+ ions. The other statements are also true, except for statement b) which is wrong.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer?
Question Description
Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer?.
Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statements is wrong? a)An acidified solution of K2Cr2O7 liberates iodine from iodides b)In acidic solution, dichromate ions are converted to chromate ions c)Ammonium dichromate on heating undergoes exothermic decomposition to give Cr2O3 d)Potassium dichromate is used as a titrant for Fe2+ ionsCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















