Class 12 Exam > Class 12 Questions > Which one of the following statements is not ...
Start Learning for Free
Which one of the following statements is not true?
- a)Buna S is a copolymer of butadiene and styrene
- b)Natral rubber is a 1,4 polymer of isoprene
- c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger
- d)Natural rubber has the trans-configuration at every double bond
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which one of the following statements is not true? a)Buna S is a copol...
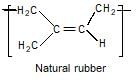
Natural rubber is cis-configuration of 1,4-polyisoprene or
Most Upvoted Answer
Which one of the following statements is not true? a)Buna S is a copol...
Explanation:
Natural rubber is a polymer of isoprene, not butadiene.
Here is an explanation of each statement:
a) Buna S is a copolymer of butadiene and styrene:
Buna S is a synthetic rubber that is a copolymer of butadiene and styrene. It is formed by the polymerization of these two monomers. This statement is true.
b) Natural rubber is a 1,4 polymer of isoprene:
Natural rubber is a polymer of isoprene. Isoprene is a monomer with a molecular formula of C5H8. In the polymerization process, isoprene molecules combine with each other in a 1,4 fashion, meaning that one carbon atom from each monomer is linked to the fourth carbon atom of the adjacent monomer. This statement is true.
c) In vulcanization, the formation of sulfur bridges between different chains makes rubber harder and stronger:
Vulcanization is a chemical process that involves the addition of sulfur to rubber. During vulcanization, sulfur bridges are formed between the polymer chains, which crosslink them and make the rubber harder, stronger, and more durable. This statement is true.
d) Natural rubber has the trans-configuration at every double bond:
This statement is not true. Natural rubber has a cis-configuration at every double bond. In the cis-configuration, the substituent groups are on the same side of the double bond, whereas in the trans-configuration, the substituent groups are on opposite sides of the double bond. The presence of the cis-configuration gives natural rubber its unique properties, such as high elasticity and flexibility.
In summary, the statement that is not true is option 'D'. Natural rubber has the cis-configuration at every double bond, not the trans-configuration.
Natural rubber is a polymer of isoprene, not butadiene.
Here is an explanation of each statement:
a) Buna S is a copolymer of butadiene and styrene:
Buna S is a synthetic rubber that is a copolymer of butadiene and styrene. It is formed by the polymerization of these two monomers. This statement is true.
b) Natural rubber is a 1,4 polymer of isoprene:
Natural rubber is a polymer of isoprene. Isoprene is a monomer with a molecular formula of C5H8. In the polymerization process, isoprene molecules combine with each other in a 1,4 fashion, meaning that one carbon atom from each monomer is linked to the fourth carbon atom of the adjacent monomer. This statement is true.
c) In vulcanization, the formation of sulfur bridges between different chains makes rubber harder and stronger:
Vulcanization is a chemical process that involves the addition of sulfur to rubber. During vulcanization, sulfur bridges are formed between the polymer chains, which crosslink them and make the rubber harder, stronger, and more durable. This statement is true.
d) Natural rubber has the trans-configuration at every double bond:
This statement is not true. Natural rubber has a cis-configuration at every double bond. In the cis-configuration, the substituent groups are on the same side of the double bond, whereas in the trans-configuration, the substituent groups are on opposite sides of the double bond. The presence of the cis-configuration gives natural rubber its unique properties, such as high elasticity and flexibility.
In summary, the statement that is not true is option 'D'. Natural rubber has the cis-configuration at every double bond, not the trans-configuration.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer?
Question Description
Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer?.
Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following statements is not true? a)Buna S is a copolymer of butadiene and styrene b)Natral rubber is a 1,4 polymer of isoprene c)In vulcanization the formation of sulphur bridges between different chains makes rubber harder and stronger d)Natural rubber has the trans-configuration at every double bondCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















