Class 12 Exam > Class 12 Questions > While extracting an element form its ore, the...
Start Learning for Free
While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]
- a)Lead
- b)Chromium
- c)Mangan ese
- d)Silver
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
While extracting an element form its ore, the ore is ground and leache...
Cyanide process is used in the metallurgy of Ag
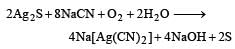
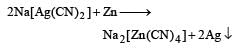
Most Upvoted Answer
While extracting an element form its ore, the ore is ground and leache...
The process described in the question is known as cyanide leaching, and it is commonly used to extract silver from its ore. Let's break down the steps involved in this process:
1. Grinding the ore: The ore containing silver is first crushed and ground into fine particles. This increases the surface area of the ore, allowing for better contact between the ore particles and the leaching solution.
2. Leaching with dilute potassium cyanide solution: The ground ore is then mixed with a dilute solution of potassium cyanide. Potassium cyanide (KCN) is a highly toxic compound that forms a complex with silver called potassium argento cyanide (KAg(CN)2).
3. Formation of potassium argento cyanide: In the presence of potassium cyanide, the silver in the ore reacts to form potassium argento cyanide, which is soluble in water. This reaction can be represented by the following equation:
Ag + 2KCN + H2O → KAg(CN)2 + KOH
4. Separation of silver: The resulting solution containing potassium argento cyanide is then separated from the remaining solid ore particles. This can be done by filtration or other separation techniques.
5. Recovery of silver from the solution: To recover silver from the potassium argento cyanide solution, various methods can be employed. One common method involves the addition of metallic zinc to the solution. The zinc reacts with the potassium argento cyanide, displacing the silver and forming solid silver particles. These particles can then be collected and further processed to obtain pure silver.
Therefore, based on the given information, the element being extracted from its ore using cyanide leaching is silver (Ag).
1. Grinding the ore: The ore containing silver is first crushed and ground into fine particles. This increases the surface area of the ore, allowing for better contact between the ore particles and the leaching solution.
2. Leaching with dilute potassium cyanide solution: The ground ore is then mixed with a dilute solution of potassium cyanide. Potassium cyanide (KCN) is a highly toxic compound that forms a complex with silver called potassium argento cyanide (KAg(CN)2).
3. Formation of potassium argento cyanide: In the presence of potassium cyanide, the silver in the ore reacts to form potassium argento cyanide, which is soluble in water. This reaction can be represented by the following equation:
Ag + 2KCN + H2O → KAg(CN)2 + KOH
4. Separation of silver: The resulting solution containing potassium argento cyanide is then separated from the remaining solid ore particles. This can be done by filtration or other separation techniques.
5. Recovery of silver from the solution: To recover silver from the potassium argento cyanide solution, various methods can be employed. One common method involves the addition of metallic zinc to the solution. The zinc reacts with the potassium argento cyanide, displacing the silver and forming solid silver particles. These particles can then be collected and further processed to obtain pure silver.
Therefore, based on the given information, the element being extracted from its ore using cyanide leaching is silver (Ag).

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer?
Question Description
While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer?.
While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer?.
Solutions for While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice While extracting an element form its ore, the ore is ground and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is [1989]a)Leadb)Chromiumc)Mangan esed)SilverCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















