Class 12 Exam > Class 12 Questions > Which one of the following substance is used ...
Start Learning for Free
Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]
- a)Phosphorus pentoxide
- b)Active charcoal
- c)Anhydrous calcium chloride
- d)Na3PO4.
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Which one of the following substance is used in the laboratory for fas...
Phosphorus pentoxide has great affinity for water. It forms ortho phosphoric acid on absorbing water
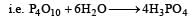
It is thus used as a powerful dehydrating or drying agent.
Most Upvoted Answer
Which one of the following substance is used in the laboratory for fas...
Substance used for fast drying of neutral gases
Phosphorus pentoxide is used in the laboratory for fast drying of neutral gases. Let's understand why:
Properties of Phosphorus pentoxide
- Phosphorus pentoxide is a powerful desiccant. It can absorb moisture from the air and react with it to form phosphoric acid.
- It is a white, crystalline solid that is commonly used as a dehydrating agent.
- It has a very high affinity for water and can absorb it from the air, making it useful for drying gases.
- It can also react with other compounds to remove water from them.
Fast drying of neutral gases
- In the laboratory, it is important to have dry gases for certain experiments.
- Phosphorus pentoxide can be used to dry neutral gases such as nitrogen, helium, and argon quickly and efficiently.
- The gas is passed through a tube containing phosphorus pentoxide, which absorbs any moisture present in the gas.
- The dry gas can then be collected and used for experiments.
Conclusion
Phosphorus pentoxide is a powerful desiccant that can be used in the laboratory for fast drying of neutral gases. Its high affinity for water allows it to absorb moisture from the air and react with it to form phosphoric acid, making it an effective dehydrating agent. It is commonly used to dry gases such as nitrogen, helium, and argon to ensure that they are dry and suitable for certain experiments.
Phosphorus pentoxide is used in the laboratory for fast drying of neutral gases. Let's understand why:
Properties of Phosphorus pentoxide
- Phosphorus pentoxide is a powerful desiccant. It can absorb moisture from the air and react with it to form phosphoric acid.
- It is a white, crystalline solid that is commonly used as a dehydrating agent.
- It has a very high affinity for water and can absorb it from the air, making it useful for drying gases.
- It can also react with other compounds to remove water from them.
Fast drying of neutral gases
- In the laboratory, it is important to have dry gases for certain experiments.
- Phosphorus pentoxide can be used to dry neutral gases such as nitrogen, helium, and argon quickly and efficiently.
- The gas is passed through a tube containing phosphorus pentoxide, which absorbs any moisture present in the gas.
- The dry gas can then be collected and used for experiments.
Conclusion
Phosphorus pentoxide is a powerful desiccant that can be used in the laboratory for fast drying of neutral gases. Its high affinity for water allows it to absorb moisture from the air and react with it to form phosphoric acid, making it an effective dehydrating agent. It is commonly used to dry gases such as nitrogen, helium, and argon to ensure that they are dry and suitable for certain experiments.

|
Explore Courses for Class 12 exam
|

|
Question Description
Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer?.
Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer?.
Solutions for Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]a)Phosphorus pentoxideb)Active charcoalc)Anhydrous calcium chlorided)Na3PO4.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















