Class 12 Exam > Class 12 Questions > When a buried pipeline is protected from corr...
Start Learning for Free
When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known as
- a)sacrified cathodic protection
- b)sacrified anodic protection
- c)impressed voltage protection
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
When a buried pipeline is protected from corrosion by connecting to Mg...
Pipes are made of Iron. Iron can be oxidised toFe2+.
Fe → Fe2+ + 2e-
but when connected to Mg, which is more electropositive that Fe, Mg is oxidised to Mg2+ and in turn reduces Fe2+ (rust) to Fe
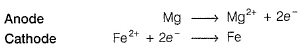
It is called cathodic protection but at the cost of Mg (sacrified anode).
Fe → Fe2+ + 2e-
but when connected to Mg, which is more electropositive that Fe, Mg is oxidised to Mg2+ and in turn reduces Fe2+ (rust) to Fe
It is called cathodic protection but at the cost of Mg (sacrified anode).
Most Upvoted Answer
When a buried pipeline is protected from corrosion by connecting to Mg...
Sacrificial Cathodic Protection
When a buried pipeline is protected from corrosion by connecting it to a magnesium (Mg) block, it is known as sacrificial cathodic protection. This method involves the use of a more reactive metal, such as magnesium, which is connected to the pipeline as an anode. The pipeline itself acts as the cathode.
Working Principle
The principle behind sacrificial cathodic protection is based on the galvanic series. The galvanic series is a list of metals and alloys arranged in order of their corrosion potential in a given environment. When two different metals are in contact with an electrolyte (e.g., soil or water), a galvanic cell is formed, and a flow of electrons occurs between the anode (more reactive metal) and the cathode (less reactive metal).
Corrosion Prevention
When the magnesium anode is connected to the buried pipeline, it undergoes oxidation (corrosion) to protect the pipeline from corrosion. The more reactive magnesium metal corrodes sacrificially, releasing electrons. These electrons flow through the conductive path provided by the pipeline, preventing the corrosion of the pipeline itself. As a result, the magnesium anode is consumed over time, sacrificing itself to protect the pipeline.
Buried Pipeline Protection
Sacrificial cathodic protection is commonly used to protect buried pipelines from corrosion. The magnesium anode is buried in close proximity to the pipeline, ensuring a good electrical connection. The anode is periodically replaced as it is consumed over time.
Advantages and Disadvantages
Advantages of sacrificial cathodic protection include:
- Simple and cost-effective method
- Does not require an external power source
- Effective in protecting a large surface area
However, there are also some limitations:
- The sacrificial anode needs to be replaced periodically
- Requires careful selection of the anode material based on the environment
- May not be suitable for all types of structures or environments
Conclusion
Sacrificial cathodic protection is an effective method for protecting buried pipelines from corrosion. By connecting a more reactive metal (such as magnesium) to the pipeline as an anode, the sacrificial cathodic protection system ensures that the pipeline remains the cathode and is protected from corrosion. This method is widely used due to its simplicity and cost-effectiveness.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer?
Question Description
When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer?.
When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer?.
Solutions for When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When a buried pipeline is protected from corrosion by connecting to Mg block ,It is known asa)sacrified cathodic protectionb)sacrified anodic protectionc)impressed voltage protectiond)None of the aboveCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















