Class 12 Exam > Class 12 Questions > A secondary amine cannot be prepared directly...
Start Learning for Free
A secondary amine cannot be prepared directly by
- a)reduction of amide
- b)reduction of cyanide
- c)reduction of imine
- d)reduction of isocyanide
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
A secondary amine cannot be prepared directly bya)reduction of amideb)...
Alkyl cyanide gives primary amine on reduction.
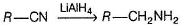
Most Upvoted Answer
A secondary amine cannot be prepared directly bya)reduction of amideb)...
Reduction of cyanide
The reduction of cyanide is a method used to prepare secondary amines. Cyanide (CN-) can be reduced to an intermediate called an imine, which can then be further reduced to form a secondary amine. Therefore, the correct answer is not option 'B'.
Reduction of amide
The reduction of amide is a common method used to prepare amines. Amides can be reduced to primary amines by using reducing agents such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4). Primary amines can further react to form secondary amines through various methods such as reductive amination.
Reduction of imine
The reduction of imine is another method to prepare secondary amines. Imine is an organic compound that contains a carbon-nitrogen double bond. It can be reduced by using reducing agents such as sodium borohydride or hydrogen gas in the presence of a metal catalyst to form a secondary amine.
Reduction of isocyanide
The reduction of isocyanide is a method used to prepare primary amines. Isocyanides can be reduced by using reducing agents such as lithium aluminum hydride or sodium borohydride to form primary amines.
Conclusion
Therefore, the correct answer is option 'B' because the reduction of cyanide can indeed be used to prepare secondary amines. The reduction of amide, imine, and isocyanide can also be used to prepare amines, but the question specifically asks for the method that cannot be used to prepare secondary amines.
The reduction of cyanide is a method used to prepare secondary amines. Cyanide (CN-) can be reduced to an intermediate called an imine, which can then be further reduced to form a secondary amine. Therefore, the correct answer is not option 'B'.
Reduction of amide
The reduction of amide is a common method used to prepare amines. Amides can be reduced to primary amines by using reducing agents such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4). Primary amines can further react to form secondary amines through various methods such as reductive amination.
Reduction of imine
The reduction of imine is another method to prepare secondary amines. Imine is an organic compound that contains a carbon-nitrogen double bond. It can be reduced by using reducing agents such as sodium borohydride or hydrogen gas in the presence of a metal catalyst to form a secondary amine.
Reduction of isocyanide
The reduction of isocyanide is a method used to prepare primary amines. Isocyanides can be reduced by using reducing agents such as lithium aluminum hydride or sodium borohydride to form primary amines.
Conclusion
Therefore, the correct answer is option 'B' because the reduction of cyanide can indeed be used to prepare secondary amines. The reduction of amide, imine, and isocyanide can also be used to prepare amines, but the question specifically asks for the method that cannot be used to prepare secondary amines.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer?
Question Description
A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer?.
A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer?.
Solutions for A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A secondary amine cannot be prepared directly bya)reduction of amideb)reduction of cyanidec)reduction of imined)reduction of isocyanideCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















