Class 12 Exam > Class 12 Questions > If all carbonyls isomers of molecular mass = ...
Start Learning for Free
If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?
Correct answer is '4'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
If all carbonyls isomers of molecular mass = 86 u are separately treat...
The carbonyl C5H10O (CnH2nO = 86) has following isomers.
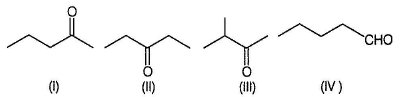

Compounds (III), (IV), (VI) and (VII) gives racemic mixture with CH3MgBr. (I) and (II) gives achiral alcohols with CH3MgBr. (V) is enantiomeric, a pure enantiomer it gives pair of diastereomers with CH3MgBr.
Compounds (III), (IV), (VI) and (VII) gives racemic mixture with CH3MgBr. (I) and (II) gives achiral alcohols with CH3MgBr. (V) is enantiomeric, a pure enantiomer it gives pair of diastereomers with CH3MgBr.
Most Upvoted Answer
If all carbonyls isomers of molecular mass = 86 u are separately treat...
Carbonyl compounds are organic compounds that contain a carbonyl group, which is a carbon atom double bonded to an oxygen atom. Isomers are compounds with the same molecular formula but different structural arrangements. In this case, we are looking for carbonyl isomers with a molecular mass of 86 u.
To determine the number of isomers that will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis, we need to consider the presence of a chiral center in the carbonyl compound.
1. Introduction:
We are given carbonyl isomers with a molecular mass of 86 u.
We need to determine the number of isomers that will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis.
2. Chiral Center:
A chiral center is an atom in a molecule that is bonded to four different groups. When a chiral center is present, two enantiomers (mirror-image isomers) can form, which would result in a racemic mixture.
3. Calculation:
To calculate the number of isomers that will give a racemic mixture, we need to consider the possible arrangements of atoms around the chiral center.
Since the molecular mass is given as 86 u, we can consider possible combinations of atoms that would result in this mass.
One possible combination is a carbonyl compound with a molecular formula of C5H7O. We can arrange the atoms in different ways to form isomers.
The possible isomers are:
- 2-pentanone (molecular mass = 86 u)
- 3-pentanone (molecular mass = 86 u)
- 4-methyl-2-pentanone (molecular mass = 86 u)
- 2-methyl-3-pentanone (molecular mass = 86 u)
Out of these four isomers, 2-pentanone and 3-pentanone do not have a chiral center, so they will not give a racemic mixture.
However, 4-methyl-2-pentanone and 2-methyl-3-pentanone have a chiral center at the carbon bonded to the carbonyl group. These two isomers will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis.
Therefore, the correct answer is '4' isomers that will give a racemic mixture.
4. Conclusion:
Out of the four carbonyl isomers with a molecular mass of 86 u, 4-methyl-2-pentanone and 2-methyl-3-pentanone have a chiral center and will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis. The other two isomers, 2-pentanone and 3-pentanone, do not have a chiral center and will not give a racemic mixture.
To determine the number of isomers that will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis, we need to consider the presence of a chiral center in the carbonyl compound.
1. Introduction:
We are given carbonyl isomers with a molecular mass of 86 u.
We need to determine the number of isomers that will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis.
2. Chiral Center:
A chiral center is an atom in a molecule that is bonded to four different groups. When a chiral center is present, two enantiomers (mirror-image isomers) can form, which would result in a racemic mixture.
3. Calculation:
To calculate the number of isomers that will give a racemic mixture, we need to consider the possible arrangements of atoms around the chiral center.
Since the molecular mass is given as 86 u, we can consider possible combinations of atoms that would result in this mass.
One possible combination is a carbonyl compound with a molecular formula of C5H7O. We can arrange the atoms in different ways to form isomers.
The possible isomers are:
- 2-pentanone (molecular mass = 86 u)
- 3-pentanone (molecular mass = 86 u)
- 4-methyl-2-pentanone (molecular mass = 86 u)
- 2-methyl-3-pentanone (molecular mass = 86 u)
Out of these four isomers, 2-pentanone and 3-pentanone do not have a chiral center, so they will not give a racemic mixture.
However, 4-methyl-2-pentanone and 2-methyl-3-pentanone have a chiral center at the carbon bonded to the carbonyl group. These two isomers will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis.
Therefore, the correct answer is '4' isomers that will give a racemic mixture.
4. Conclusion:
Out of the four carbonyl isomers with a molecular mass of 86 u, 4-methyl-2-pentanone and 2-methyl-3-pentanone have a chiral center and will give a racemic mixture when treated with CH3MgBr followed by acid hydrolysis. The other two isomers, 2-pentanone and 3-pentanone, do not have a chiral center and will not give a racemic mixture.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer?
Question Description
If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer?.
If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer?.
Solutions for If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer?, a detailed solution for If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? has been provided alongside types of If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If all carbonyls isomers of molecular mass = 86 u are separately treated with CH3MgBr followed by acid hydrolysis, how many of them will give racemic mixture ?Correct answer is '4'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















