Class 9 Exam > Class 9 Questions > What is hypertonic, hypotonic and isotonic so...
Start Learning for Free
What is hypertonic, hypotonic and isotonic solution?
Verified Answer
What is hypertonic, hypotonic and isotonic solution?
Isotonic, Hypotonic, and Hypertonic Solutions
Water moves readily across cell membranes through special protein-lined channels, and if the total concentration of all dissolved solutes is not equal on both sides, there will be net movement of water molecules into or out of the cell. Whether there is net movement of water into or out of the cell and which direction it moves depends on whether the cell’s environment is isotonic, hypotonic, or hypertonic.
 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
What is hypertonic, hypotonic and isotonic solution?
If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. In an isotonic environment, the relative concentrations of solute and water are equal on both sides of the membrane. ... When a cell is placed in a hypotonic environment, water will enter the cell, and the cell will swell.
Community Answer
What is hypertonic, hypotonic and isotonic solution?
Hypertonic, Hypotonic, and Isotonic Solutions: An Overview
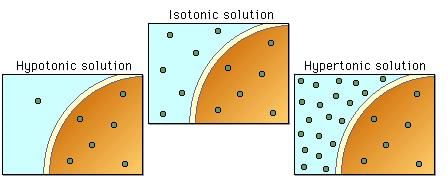
Hypertonic Solution
A hypertonic solution refers to a solution that has a higher concentration of solutes compared to another solution. In other words, it has a higher osmolarity than the solution it is being compared to. When a cell is placed in a hypertonic solution, water tends to move out of the cell to balance the concentration gradient. This can cause the cell to shrink or undergo a process called crenation.
Hypotonic Solution
On the other hand, a hypotonic solution has a lower concentration of solutes compared to another solution. It has a lower osmolarity than the solution it is being compared to. When a cell is placed in a hypotonic solution, water moves into the cell to balance the concentration gradient. This can cause the cell to swell or even burst, a process called lysis.
Isotonic Solution
An isotonic solution has the same concentration of solutes as the solution it is being compared to. It has the same osmolarity. When a cell is placed in an isotonic solution, there is no net movement of water across the cell membrane. This means that the cell maintains its shape and size without undergoing any significant changes.
Key Points:
1. Hypertonic solution: Higher solute concentration, water moves out of the cell, causes cell shrinkage (crenation).
2. Hypotonic solution: Lower solute concentration, water moves into the cell, causes cell swelling or bursting (lysis).
3. Isotonic solution: Same solute concentration, no net movement of water, cell maintains its shape and size.
Effects on Cells:
- Hypertonic solutions cause water to move out of cells, leading to cell shrinkage. This can be seen in red blood cells placed in a concentrated saline solution.
- Hypotonic solutions cause water to move into cells, leading to cell swelling or even bursting. This can be observed when plant cells are placed in distilled water.
- Isotonic solutions maintain the equilibrium of water movement, keeping the cell shape and size stable. This is the condition in which most animal cells function optimally.
Applications:
- Hypertonic solutions can be used in medical settings to draw excess water out of swollen tissues, reducing edema.
- Hypotonic solutions are commonly used in intravenous fluids to provide hydration and prevent dehydration.
- Isotonic solutions, such as normal saline, are used for various purposes, including rehydration and restoring electrolyte balance.
Understanding these concepts of hypertonic, hypotonic, and isotonic solutions is crucial in various biological and medical contexts as it helps explain the effects of different solutions on cells and their applications in practical settings.
Hypertonic Solution
A hypertonic solution refers to a solution that has a higher concentration of solutes compared to another solution. In other words, it has a higher osmolarity than the solution it is being compared to. When a cell is placed in a hypertonic solution, water tends to move out of the cell to balance the concentration gradient. This can cause the cell to shrink or undergo a process called crenation.
Hypotonic Solution
On the other hand, a hypotonic solution has a lower concentration of solutes compared to another solution. It has a lower osmolarity than the solution it is being compared to. When a cell is placed in a hypotonic solution, water moves into the cell to balance the concentration gradient. This can cause the cell to swell or even burst, a process called lysis.
Isotonic Solution
An isotonic solution has the same concentration of solutes as the solution it is being compared to. It has the same osmolarity. When a cell is placed in an isotonic solution, there is no net movement of water across the cell membrane. This means that the cell maintains its shape and size without undergoing any significant changes.
Key Points:
1. Hypertonic solution: Higher solute concentration, water moves out of the cell, causes cell shrinkage (crenation).
2. Hypotonic solution: Lower solute concentration, water moves into the cell, causes cell swelling or bursting (lysis).
3. Isotonic solution: Same solute concentration, no net movement of water, cell maintains its shape and size.
Effects on Cells:
- Hypertonic solutions cause water to move out of cells, leading to cell shrinkage. This can be seen in red blood cells placed in a concentrated saline solution.
- Hypotonic solutions cause water to move into cells, leading to cell swelling or even bursting. This can be observed when plant cells are placed in distilled water.
- Isotonic solutions maintain the equilibrium of water movement, keeping the cell shape and size stable. This is the condition in which most animal cells function optimally.
Applications:
- Hypertonic solutions can be used in medical settings to draw excess water out of swollen tissues, reducing edema.
- Hypotonic solutions are commonly used in intravenous fluids to provide hydration and prevent dehydration.
- Isotonic solutions, such as normal saline, are used for various purposes, including rehydration and restoring electrolyte balance.
Understanding these concepts of hypertonic, hypotonic, and isotonic solutions is crucial in various biological and medical contexts as it helps explain the effects of different solutions on cells and their applications in practical settings.
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
What is hypertonic, hypotonic and isotonic solution?
Question Description
What is hypertonic, hypotonic and isotonic solution? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about What is hypertonic, hypotonic and isotonic solution? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is hypertonic, hypotonic and isotonic solution?.
What is hypertonic, hypotonic and isotonic solution? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about What is hypertonic, hypotonic and isotonic solution? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is hypertonic, hypotonic and isotonic solution?.
Solutions for What is hypertonic, hypotonic and isotonic solution? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of What is hypertonic, hypotonic and isotonic solution? defined & explained in the simplest way possible. Besides giving the explanation of
What is hypertonic, hypotonic and isotonic solution?, a detailed solution for What is hypertonic, hypotonic and isotonic solution? has been provided alongside types of What is hypertonic, hypotonic and isotonic solution? theory, EduRev gives you an
ample number of questions to practice What is hypertonic, hypotonic and isotonic solution? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























