Mechanical Engineering Exam > Mechanical Engineering Questions > Which one of the following is correct? The ca...
Start Learning for Free
Which one of the following is correct? The capillary rise on depression in a small diameter tube is
- a)Directly proportional to the specific weight of the fluid
- b)Inversely proportional to the surface tension
- c)Inversely proportional to the diameter
- d)Directly proportional to the surface area
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Which one of the following is correct? The capillary rise on depressio...
Ans. (c) The capillary rise on depression is given by,
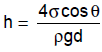
 This question is part of UPSC exam. View all Mechanical Engineering courses
This question is part of UPSC exam. View all Mechanical Engineering courses
Most Upvoted Answer
Which one of the following is correct? The capillary rise on depressio...
Capillary Rise on Depression in a Small Diameter Tube
The correct answer is option 'C': The capillary rise on depression in a small diameter tube is inversely proportional to the diameter. Let's understand why this is the correct answer.
Capillary Rise
Capillary rise refers to the phenomenon of a liquid rising or being depressed in a narrow tube or capillary. It occurs due to the intermolecular forces between the liquid molecules and the solid surface of the tube.
Influence of Tube Diameter
The capillary rise is inversely proportional to the diameter of the tube. This means that as the diameter of the tube decreases, the capillary rise increases. Conversely, as the diameter of the tube increases, the capillary rise decreases.
Surface Tension
Surface tension is the property of a liquid's surface that allows it to resist external forces. It is caused by the cohesive forces between the liquid molecules. Surface tension plays a significant role in capillary rise.
Influence of Surface Tension
The capillary rise is directly proportional to the surface tension of the liquid. This means that as the surface tension of the liquid increases, the capillary rise also increases. Similarly, if the surface tension decreases, the capillary rise decreases.
Relationship between Diameter and Capillary Rise
The relationship between the tube diameter and capillary rise can be explained by considering the forces involved. The capillary rise is caused by the balance between the adhesive forces between the liquid and the tube and the cohesive forces within the liquid.
When the tube diameter is small, the adhesive forces dominate over the cohesive forces, leading to a higher capillary rise. This is because the liquid molecules experience a higher attraction to the solid surface of the tube. As a result, the liquid rises or is depressed more in the tube.
On the other hand, when the tube diameter is large, the cohesive forces within the liquid become more significant compared to the adhesive forces. This reduces the capillary rise as the liquid molecules experience less attraction to the tube surface.
Therefore, the capillary rise is inversely proportional to the diameter of the tube. A smaller tube diameter results in a higher capillary rise, while a larger tube diameter leads to a lower capillary rise.
Conclusion
In summary, the capillary rise on depression in a small diameter tube is inversely proportional to the diameter. This relationship is due to the dominance of adhesive forces over cohesive forces in a small diameter tube, resulting in a higher capillary rise.
The correct answer is option 'C': The capillary rise on depression in a small diameter tube is inversely proportional to the diameter. Let's understand why this is the correct answer.
Capillary Rise
Capillary rise refers to the phenomenon of a liquid rising or being depressed in a narrow tube or capillary. It occurs due to the intermolecular forces between the liquid molecules and the solid surface of the tube.
Influence of Tube Diameter
The capillary rise is inversely proportional to the diameter of the tube. This means that as the diameter of the tube decreases, the capillary rise increases. Conversely, as the diameter of the tube increases, the capillary rise decreases.
Surface Tension
Surface tension is the property of a liquid's surface that allows it to resist external forces. It is caused by the cohesive forces between the liquid molecules. Surface tension plays a significant role in capillary rise.
Influence of Surface Tension
The capillary rise is directly proportional to the surface tension of the liquid. This means that as the surface tension of the liquid increases, the capillary rise also increases. Similarly, if the surface tension decreases, the capillary rise decreases.
Relationship between Diameter and Capillary Rise
The relationship between the tube diameter and capillary rise can be explained by considering the forces involved. The capillary rise is caused by the balance between the adhesive forces between the liquid and the tube and the cohesive forces within the liquid.
When the tube diameter is small, the adhesive forces dominate over the cohesive forces, leading to a higher capillary rise. This is because the liquid molecules experience a higher attraction to the solid surface of the tube. As a result, the liquid rises or is depressed more in the tube.
On the other hand, when the tube diameter is large, the cohesive forces within the liquid become more significant compared to the adhesive forces. This reduces the capillary rise as the liquid molecules experience less attraction to the tube surface.
Therefore, the capillary rise is inversely proportional to the diameter of the tube. A smaller tube diameter results in a higher capillary rise, while a larger tube diameter leads to a lower capillary rise.
Conclusion
In summary, the capillary rise on depression in a small diameter tube is inversely proportional to the diameter. This relationship is due to the dominance of adhesive forces over cohesive forces in a small diameter tube, resulting in a higher capillary rise.
Attention Mechanical Engineering Students!
To make sure you are not studying endlessly, EduRev has designed Mechanical Engineering study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Mechanical Engineering.

|
Explore Courses for Mechanical Engineering exam
|

|
Similar Mechanical Engineering Doubts
Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer?.
Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following is correct? The capillary rise on depression in a small diameter tube isa)Directly proportional to the specific weight of the fluidb)Inversely proportional to the surface tensionc)Inversely proportional to the diameterd)Directly proportional to the surface areaCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























