JEE Exam > JEE Questions > Statement-1 : The geometrical isomers of the ...
Start Learning for Free
Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. and
Statement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.
- a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.
- b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1
- c)Statement-1 is True, Statement-2 is False
- d)Statement-1 is False, Statement-2 is True
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are ...
The geometrical isomers of [M(NH3)4Cl2] can be represented as follows:-
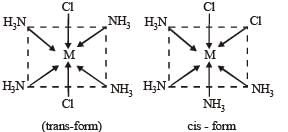
These isomers are optically inactive and they posses axis of symmetry.
Both the statements are thus true. Out of two possible answers i.e. option (a) and (b) option (b) is correct as the statement 2 is not a correct explanation of statement 1.
For a molecule to be optically active it should not possess alternate axis of symmetry.
Both the statements are thus true. Out of two possible answers i.e. option (a) and (b) option (b) is correct as the statement 2 is not a correct explanation of statement 1.
For a molecule to be optically active it should not possess alternate axis of symmetry.
Most Upvoted Answer
Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are ...
The correct answer is option B
Statement-1: The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive.
Statement-2: Both geometrical isomers of the complex [M(NH3)4Cl2] possess an axis of symmetry.
Explanation:
Geometrical isomerism: It occurs in coordination compounds when two or more different ligands are attached to the central metal ion in different positions. This results in different spatial arrangements of the ligands around the metal ion. These different spatial arrangements are called geometrical isomers.
Optical activity: Optical activity is observed in compounds that are chiral, meaning they have a non-superimposable mirror image. Chiral compounds rotate the plane of polarized light.
Axis of symmetry: An axis of symmetry is an imaginary line around which an object can be rotated and still appear the same. In the case of coordination compounds, an axis of symmetry can be present if the complex has a regular geometric shape.
Explanation of Statement-1:
The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. This is because the two geometrical isomers are related to each other by a plane of symmetry. A plane of symmetry is a plane that divides an object into two halves that are mirror images of each other. Since the two geometrical isomers are mirror images of each other, they cancel out each other's optical activity, resulting in the overall compound being optically inactive.
Explanation of Statement-2:
Both geometrical isomers of the complex [M(NH3)4Cl2] possess an axis of symmetry. An axis of symmetry can be present if the complex has a regular geometric shape. In the case of [M(NH3)4Cl2], both geometrical isomers have a square planar shape, which possesses a C4 axis of symmetry. The C4 axis of symmetry is an axis that can rotate the complex by 90 degrees and still appear the same. Therefore, Statement-2 is true.
Explanation of the Correct Answer:
Statement-1 is true because the geometrical isomers of the complex [M(NH3)4Cl2] are indeed optically inactive. Statement-2 is also true because both geometrical isomers possess an axis of symmetry. However, Statement-2 is not a correct explanation for Statement-1 because the presence of an axis of symmetry does not directly imply the optical inactivity of the compound. Optical activity is determined by the presence of chiral centers or lack of a plane of symmetry. In this case, the optically inactive nature of the compound is due to the presence of a plane of symmetry, not the axis of symmetry. Therefore, the correct answer is option B.
Statement-1: The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive.
Statement-2: Both geometrical isomers of the complex [M(NH3)4Cl2] possess an axis of symmetry.
Explanation:
Geometrical isomerism: It occurs in coordination compounds when two or more different ligands are attached to the central metal ion in different positions. This results in different spatial arrangements of the ligands around the metal ion. These different spatial arrangements are called geometrical isomers.
Optical activity: Optical activity is observed in compounds that are chiral, meaning they have a non-superimposable mirror image. Chiral compounds rotate the plane of polarized light.
Axis of symmetry: An axis of symmetry is an imaginary line around which an object can be rotated and still appear the same. In the case of coordination compounds, an axis of symmetry can be present if the complex has a regular geometric shape.
Explanation of Statement-1:
The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. This is because the two geometrical isomers are related to each other by a plane of symmetry. A plane of symmetry is a plane that divides an object into two halves that are mirror images of each other. Since the two geometrical isomers are mirror images of each other, they cancel out each other's optical activity, resulting in the overall compound being optically inactive.
Explanation of Statement-2:
Both geometrical isomers of the complex [M(NH3)4Cl2] possess an axis of symmetry. An axis of symmetry can be present if the complex has a regular geometric shape. In the case of [M(NH3)4Cl2], both geometrical isomers have a square planar shape, which possesses a C4 axis of symmetry. The C4 axis of symmetry is an axis that can rotate the complex by 90 degrees and still appear the same. Therefore, Statement-2 is true.
Explanation of the Correct Answer:
Statement-1 is true because the geometrical isomers of the complex [M(NH3)4Cl2] are indeed optically inactive. Statement-2 is also true because both geometrical isomers possess an axis of symmetry. However, Statement-2 is not a correct explanation for Statement-1 because the presence of an axis of symmetry does not directly imply the optical inactivity of the compound. Optical activity is determined by the presence of chiral centers or lack of a plane of symmetry. In this case, the optically inactive nature of the compound is due to the presence of a plane of symmetry, not the axis of symmetry. Therefore, the correct answer is option B.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer?
Question Description
Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer?.
Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Statement-1 : The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive. andStatement-2 : Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.a)Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1.b)Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1c)Statement-1 is True, Statement-2 is Falsed)Statement-1 is False, Statement-2 is TrueCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























