JEE Exam > JEE Questions > On moving across a period, the basic characte...
Start Learning for Free
On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.
(1) SO3 (2) Cl2O7 (3) N2O5 (4) CO2
Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.
(1) SO3 (2) Cl2O7 (3) N2O5 (4) CO2
Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.
- a)a
- b)b
- c)c
- d)d
Correct answer is '4312'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
On moving across a period, the basic character of the oxides gradually...
Acidic Character of oxides increase as non-metallic character of the element that is combined with oxygen increase.
The ionisation energy is the measure of matallic and non-metallic character
AS ionisation energy of element decreases the matallic character increases and non-metallic charachter decreases and vice-versa.
Hence the correct increasing order of acidic character is increasing non-metallic character
The ionisation energy is the measure of matallic and non-metallic character
AS ionisation energy of element decreases the matallic character increases and non-metallic charachter decreases and vice-versa.
Hence the correct increasing order of acidic character is increasing non-metallic character
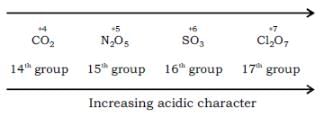
Most Upvoted Answer
On moving across a period, the basic character of the oxides gradually...
Explanation:
When it comes to the acidic character of oxides, it is determined by the ability of the oxide to donate protons (H+) in aqueous solution. The more easily an oxide can donate protons, the stronger its acidic character.
In general, the acidic character of oxides increases from left to right across a period. This is because as you move across a period, the electronegativity of the elements increases, leading to a stronger pull on the shared electrons in the oxide. This results in a higher polarity of the oxide and a greater ability to donate protons.
Now, let's analyze each of the given oxides and arrange them in order of increasing acidic character:
(1) SO3: Sulfur trioxide is an acidic oxide. It reacts with water to form sulfuric acid (H2SO4). Since it is already an acidic oxide, it will have the highest acidic character among the given options.
(2) Cl2O7: Dichlorine heptoxide is also an acidic oxide. It reacts with water to form perchloric acid (HClO4). Although it is less acidic than sulfur trioxide, it is more acidic than the remaining options.
(3) N2O5: Dinitrogen pentoxide is a strong acidic oxide. It reacts with water to form nitric acid (HNO3). It is less acidic than sulfur trioxide and dichlorine heptoxide.
(4) CO2: Carbon dioxide is a weakly acidic oxide. It reacts with water to form carbonic acid (H2CO3). It is the least acidic among the given options.
Therefore, the correct order of increasing acidic character is:
a) 4 (CO2)
b) 3 (N2O5)
c) 1 (SO3)
d) 2 (Cl2O7)
When it comes to the acidic character of oxides, it is determined by the ability of the oxide to donate protons (H+) in aqueous solution. The more easily an oxide can donate protons, the stronger its acidic character.
In general, the acidic character of oxides increases from left to right across a period. This is because as you move across a period, the electronegativity of the elements increases, leading to a stronger pull on the shared electrons in the oxide. This results in a higher polarity of the oxide and a greater ability to donate protons.
Now, let's analyze each of the given oxides and arrange them in order of increasing acidic character:
(1) SO3: Sulfur trioxide is an acidic oxide. It reacts with water to form sulfuric acid (H2SO4). Since it is already an acidic oxide, it will have the highest acidic character among the given options.
(2) Cl2O7: Dichlorine heptoxide is also an acidic oxide. It reacts with water to form perchloric acid (HClO4). Although it is less acidic than sulfur trioxide, it is more acidic than the remaining options.
(3) N2O5: Dinitrogen pentoxide is a strong acidic oxide. It reacts with water to form nitric acid (HNO3). It is less acidic than sulfur trioxide and dichlorine heptoxide.
(4) CO2: Carbon dioxide is a weakly acidic oxide. It reacts with water to form carbonic acid (H2CO3). It is the least acidic among the given options.
Therefore, the correct order of increasing acidic character is:
a) 4 (CO2)
b) 3 (N2O5)
c) 1 (SO3)
d) 2 (Cl2O7)
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer?
Question Description
On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer?.
On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer?.
Solutions for On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer?, a detailed solution for On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? has been provided alongside types of On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice On moving across a period, the basic character of the oxides gradually changes first into amphoteric and finally into acidic character. The acidic character of oxides increases with increase in the oxidation states of the element that is combined with oxygen. Arrange the following oxides in order of increasing acidic character.(1)SO3(2)Cl2O7(3)N2O5 (4)CO2Fill in the boxes provided below with suitable number. Least acidic oxide will come in first box and the strongest acidic oxide in the last box.a)ab)bc)cd)dCorrect answer is '4312'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.






















