Class 12 Exam > Class 12 Questions > Comprehension Type Direction (Q. Nos. 16and 1...
Start Learning for Free
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Impure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).
Q.
In the extraction of nickel by Mond’s process, the metal is obtained by
- a)electrochemical reduction
- b)thermal decomposition
- c)chemical reduction by aluminium
- d)chemical reduction by carbon
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Comprehension Type Direction (Q. Nos. 16and 17) This section contains ...
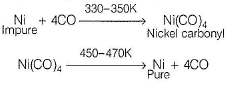
The metal is obtained by thermal decomposition. Impure nickel is heated in a current of CO at 330-350 K. It forms volatile nickel carbonyl leaving behind the impurities.
Most Upvoted Answer
Comprehension Type Direction (Q. Nos. 16and 17) This section contains ...
Understanding Monds Process for Nickel Extraction
The Monds process is a well-established method for extracting nickel from its ores, particularly when the nickel is present in a form that can be converted into a volatile compound.
Process Overview
- The Monds process primarily involves the formation of nickel carbonyl (Ni(CO)4), a gaseous compound.
- Nickel ores are first treated with carbon monoxide at elevated temperatures, leading to the formation of this volatile compound.
Thermal Decomposition
- Once nickel carbonyl is formed, it is subjected to thermal decomposition.
- When nickel carbonyl gas is heated, it breaks down into nickel metal and carbon monoxide.
- This step is crucial as it effectively separates pure nickel from impurities.
Why Option B is Correct
- The extraction of nickel in the Monds process is characterized by this thermal decomposition of nickel carbonyl.
- Other methods mentioned, such as electrochemical reduction or chemical reduction by aluminum or carbon, do not apply to this specific process, making option B the most accurate choice.
Importance of Monds Process
- This method not only yields high purity nickel but also allows for efficient processing of nickel ores with low environmental impact.
- The unique nature of nickel carbonyl's volatility is key to the efficient extraction and purification of nickel.
In summary, the Monds process utilizes thermal decomposition to extract nickel, which is why option B is the correct answer for this question.
The Monds process is a well-established method for extracting nickel from its ores, particularly when the nickel is present in a form that can be converted into a volatile compound.
Process Overview
- The Monds process primarily involves the formation of nickel carbonyl (Ni(CO)4), a gaseous compound.
- Nickel ores are first treated with carbon monoxide at elevated temperatures, leading to the formation of this volatile compound.
Thermal Decomposition
- Once nickel carbonyl is formed, it is subjected to thermal decomposition.
- When nickel carbonyl gas is heated, it breaks down into nickel metal and carbon monoxide.
- This step is crucial as it effectively separates pure nickel from impurities.
Why Option B is Correct
- The extraction of nickel in the Monds process is characterized by this thermal decomposition of nickel carbonyl.
- Other methods mentioned, such as electrochemical reduction or chemical reduction by aluminum or carbon, do not apply to this specific process, making option B the most accurate choice.
Importance of Monds Process
- This method not only yields high purity nickel but also allows for efficient processing of nickel ores with low environmental impact.
- The unique nature of nickel carbonyl's volatility is key to the efficient extraction and purification of nickel.
In summary, the Monds process utilizes thermal decomposition to extract nickel, which is why option B is the correct answer for this question.
Free Test
FREE
| Start Free Test |
Community Answer
Comprehension Type Direction (Q. Nos. 16and 17) This section contains ...
The correct option is thermal decomposition.
The nickel is heated in a stream of carbon monoxide to form a volatile complex, nickel tetra carbonyl. Later the carbonyl is subjected to higher temperature undergo decomposition giving a pure nickel.
The nickel is heated in a stream of carbon monoxide to form a volatile complex, nickel tetra carbonyl. Later the carbonyl is subjected to higher temperature undergo decomposition giving a pure nickel.

|
Explore Courses for Class 12 exam
|

|
Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer?
Question Description
Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer?.
Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Comprehension Type Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageImpure metals are purified by different methods in order to remove the undesired impurities. These methods depend on the nature of metal and nature of impurities. Liquation is used for purifying metals with low melting points like Bi, Sn, Pb, Hg etc. Distillation is used for volatile metals like Zn, Cd, Hg. Cupellation is used to purify silver metal with lead impurities. Electrolytic refining (Cu, Ag, Mg, Al) Zone refining (Si, Ge, Ga) Mond’s process (Ni) van Arkel process (Zr, Ti).Q.In the extraction of nickel by Mond’s process, the metal is obtained bya)electrochemical reductionb)thermal decompositionc)chemical reduction by aluminiumd)chemical reduction by carbonCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
















