Class 12 Exam > Class 12 Questions > PassageHydrated sodium thiosulphate, Na2S2O3....
Start Learning for Free
Passage
Hydrated sodium thiosulphate, Na2S2O3 .5H2O is called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.
Na2S + Na2SO3 + I2 → 2NaI + Na2S2O3 (Spring's reaction)
It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.
Q.
In Na2S4O6, sulphur has different oxidation states. They are
- a)+5 and +3
- b)+ 5 and 0
- c)2.5 and 2.5
- d)+ 4 and +6
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It ca...
The structure of Na2S4O6
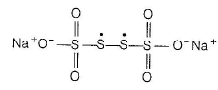
In this structure, two central sulphur atoms have zero oxidation number because electron pair forming th e S—S bond remain in the centre. Let the oxidation number of (remaining atoms) S atom be X.
2(+1)+ 6(-2)+ 2x+ 2(0) = 0
For Na for O
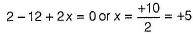
∴ Two central S-atoms have zero oxidation state and two terminal S- atoms have +5 oxidation state,
In this structure, two central sulphur atoms have zero oxidation number because electron pair forming th e S—S bond remain in the centre. Let the oxidation number of (remaining atoms) S atom be X.
2(+1)+ 6(-2)+ 2x+ 2(0) = 0
For Na for O
∴ Two central S-atoms have zero oxidation state and two terminal S- atoms have +5 oxidation state,
Most Upvoted Answer
PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It ca...
Dissolving sodium thiosulphate pentahydrate, Na2S2O3.5H2O, in water. This compound is commonly used in photography as a fixing agent to remove unexposed silver halide from photographic film or paper.
To prepare hypo, one can start by measuring a desired amount of sodium thiosulphate pentahydrate. This compound is usually available as a white crystalline solid. The amount to be measured depends on the desired concentration of the hypo solution.
Next, a suitable amount of water is heated in a container. The container should be of a size that can accommodate the measured amount of sodium thiosulphate pentahydrate and leave enough space for stirring. The water should be heated to a temperature that allows the sodium thiosulphate pentahydrate to dissolve easily.
Once the water is heated, the measured amount of sodium thiosulphate pentahydrate is added to the container and stirred continuously. The stirring helps to dissolve the crystals faster and ensures a homogeneous solution.
The stirring is continued until all the sodium thiosulphate pentahydrate has dissolved completely. At this point, the solution will appear clear and colorless.
After the sodium thiosulphate pentahydrate has dissolved, the solution is allowed to cool to room temperature. This cooling process may take some time depending on the initial temperature of the water and the quantity of sodium thiosulphate pentahydrate used.
Once the solution has cooled, it can be transferred to a suitable storage container. The hypo solution should be stored in a tightly sealed container to prevent evaporation and contamination. It is important to label the container with the name of the solution, its concentration, and the date of preparation.
In conclusion, hypo, or hydrated sodium thiosulphate, Na2S2O3.5H2O, can be prepared by dissolving sodium thiosulphate pentahydrate in water. This solution is commonly used in photography as a fixing agent. The preparation involves measuring the desired amount of sodium thiosulphate pentahydrate, dissolving it in heated water, stirring until complete dissolution, cooling the solution, and transferring it to a storage container.
To prepare hypo, one can start by measuring a desired amount of sodium thiosulphate pentahydrate. This compound is usually available as a white crystalline solid. The amount to be measured depends on the desired concentration of the hypo solution.
Next, a suitable amount of water is heated in a container. The container should be of a size that can accommodate the measured amount of sodium thiosulphate pentahydrate and leave enough space for stirring. The water should be heated to a temperature that allows the sodium thiosulphate pentahydrate to dissolve easily.
Once the water is heated, the measured amount of sodium thiosulphate pentahydrate is added to the container and stirred continuously. The stirring helps to dissolve the crystals faster and ensures a homogeneous solution.
The stirring is continued until all the sodium thiosulphate pentahydrate has dissolved completely. At this point, the solution will appear clear and colorless.
After the sodium thiosulphate pentahydrate has dissolved, the solution is allowed to cool to room temperature. This cooling process may take some time depending on the initial temperature of the water and the quantity of sodium thiosulphate pentahydrate used.
Once the solution has cooled, it can be transferred to a suitable storage container. The hypo solution should be stored in a tightly sealed container to prevent evaporation and contamination. It is important to label the container with the name of the solution, its concentration, and the date of preparation.
In conclusion, hypo, or hydrated sodium thiosulphate, Na2S2O3.5H2O, can be prepared by dissolving sodium thiosulphate pentahydrate in water. This solution is commonly used in photography as a fixing agent. The preparation involves measuring the desired amount of sodium thiosulphate pentahydrate, dissolving it in heated water, stirring until complete dissolution, cooling the solution, and transferring it to a storage container.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer?
Question Description
PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer?.
PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer?.
Solutions for PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer?, a detailed solution for PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice PassageHydrated sodium thiosulphate, Na2S2O3.5H2Ois called hypo. It can be prepared by Spring’s reaction by adding iodine to a mixture of sodium sulphide and sodium sulphite.Na2S + Na2SO3+ I2→ 2NaI + Na2S2O3(Spring's reaction)It is an efflorescent substance and at 215°C, it is completely dehydrated to Na2S2O3. Its reaction with iodine is used fo r the iodometric estimation of oxidising agents like CuSO4, CaOCI2, KMnO4, MnO2, etc., which on reaction with KI liberate I2. It is used in the bleaching industry to destroy excess chlorine on fabrics. It is used in photography as fixer since, it dissolves unexposed AgBr on the film.Q.In Na2S4O6, sulphur has different oxidation states. They area)+5 and +3b)+ 5 and 0c)2.5 and 2.5d)+ 4 and +6Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















