JEE Exam > JEE Questions > Directions: Questions are based on the follow...
Start Learning for Free
Directions: Questions are based on the following paragraph.
When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4 solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2 boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4 used in the two titrations was 5 : 1. KMnO4 oxidises all oxidation state of vanadium to Vanadium (+V) and SO2 reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.
Q. What is the oxidation state of vanadium in the compound Z?
- a)+2
- b)+1
- c)0
- d)-1
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Directions: Questions are based on the following paragraph.When ammoni...
vanadate ion 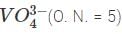 is reduce to Vx (species Z) by
is reduce to Vx (species Z) by 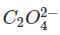
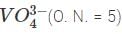 is reduce to Vx (species Z) by
is reduce to Vx (species Z) by 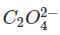
ion in acidic medium. Vx is oxidised by
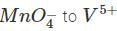
∴ 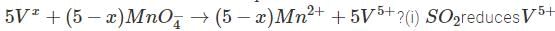 to which in turn isoxidised to
to which in turn isoxidised to 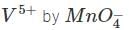
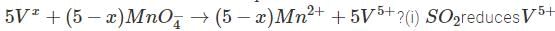 to which in turn isoxidised to
to which in turn isoxidised to 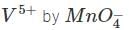
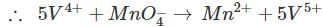 volumes of
volumes of 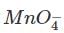 used in Eqs (1) , (2) are in ratio , of
used in Eqs (1) , (2) are in ratio , of 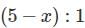
∴ 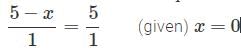
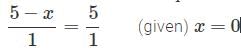
Most Upvoted Answer
Directions: Questions are based on the following paragraph.When ammoni...
Explanation:
Oxidation state of vanadium in compound Z
- In order to determine the oxidation state of vanadium in the compound Z, we must understand the reactions involved in the experiment.
- When ammonium vanadate is heated with oxalic acid solution, compound Z is formed. This reaction leads to the formation of a compound in which vanadium is in a specific oxidation state.
- The subsequent titrations with KMnO4 and SO2 indicate the oxidation and reduction states of vanadium in the compound Z.
- KMnO4 oxidizes vanadium to the +V oxidation state, while SO2 reduces vanadium from +V to +IV oxidation state.
- The ratio of volumes of KMnO4 used in the two titrations is 5:1, indicating the specific oxidation state of vanadium in compound Z.
- Since KMnO4 oxidizes vanadium to +V state and the ratio of volumes used is 5:1, it implies that the oxidation state of vanadium in compound Z is 0 (zero).
- Therefore, the correct answer is option c) 0.
By analyzing the experimental procedure and understanding the oxidation and reduction reactions involved, we can determine that the oxidation state of vanadium in compound Z is 0.
Oxidation state of vanadium in compound Z
- In order to determine the oxidation state of vanadium in the compound Z, we must understand the reactions involved in the experiment.
- When ammonium vanadate is heated with oxalic acid solution, compound Z is formed. This reaction leads to the formation of a compound in which vanadium is in a specific oxidation state.
- The subsequent titrations with KMnO4 and SO2 indicate the oxidation and reduction states of vanadium in the compound Z.
- KMnO4 oxidizes vanadium to the +V oxidation state, while SO2 reduces vanadium from +V to +IV oxidation state.
- The ratio of volumes of KMnO4 used in the two titrations is 5:1, indicating the specific oxidation state of vanadium in compound Z.
- Since KMnO4 oxidizes vanadium to +V state and the ratio of volumes used is 5:1, it implies that the oxidation state of vanadium in compound Z is 0 (zero).
- Therefore, the correct answer is option c) 0.
By analyzing the experimental procedure and understanding the oxidation and reduction reactions involved, we can determine that the oxidation state of vanadium in compound Z is 0.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer?
Question Description
Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer?.
Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer?.
Solutions for Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions: Questions are based on the following paragraph.When ammonium vanadate is heated with oxalic add solution, a compound Z is formed. A sample of Z was titrated with KMnO4solution in hot acidic solution. The resulting liquid was reduced with SO2, the excess SO2boiled off, and the liquid again titrated with KMnO4. The ratio of the volumes of KMnO4used in the two titrations was 5 : 1. KMnO4oxidises all oxidation state of vanadium to Vanadium (+V) and SO2reduces vanadium (+V) to vanadium (+IV). Read the above experiment and answer the following questions.Q.What is the oxidation state of vanadium in the compound Z?a)+2b)+1c)0d)-1Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























