Class 12 Exam > Class 12 Questions > Comprehension TypeDirection (Q. Nos. 16and 17...
Start Learning for Free
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Rutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.
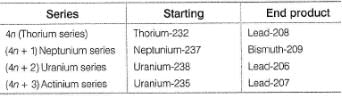
There are four disintegration series. They are
There are four disintegration series. They are
Q.
The number of alpha and beta particles emitted in the nuclear reaction 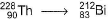 are
are
- a)4α and 1β
- b)3α and 7β
- c)8α and 1β
- d)4α and 7β
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a...
Number of alpha particles emitted
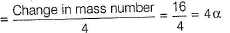
Number of beta particles liberated
= Double the alpha particles - change in atomic number
= 8 - 7 = 1
Number of beta particles liberated
= Double the alpha particles - change in atomic number
= 8 - 7 = 1

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer?
Question Description
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer?.
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Comprehension TypeDirection (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageRutherford and Soddy found that every uranium ore that they examined contained minute quantities of radium and an appreciable amount of non-radioactive lead. From this, they concluded that uranium is the starting substance in a series of radioactive transformations which pass through radium and ultimately ends with stable lead. These transformations are accompanied by spontaneous emissions of alpha and beta particles resulting in the formation of new atoms. All the nuclei from the initial element to the final stable element constitute a series called disintegration series. Mass number changes by 4 units per each alpha particle emission.There are four disintegration series. They areQ.The number of alpha and beta particles emitted in the nuclear reaction are a)4α and 1βb)3α and 7βc)8α and 1βd)4α and 7βCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















