IIT JAM Exam > IIT JAM Questions > For the hydrolysis of 1 mole of ATP at 37°...
Start Learning for Free
For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº = -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1
(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)
(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)
- a)-46.9 KJ mol-1
- b)+46.9 KJ mol-1
- c)-35 KJ mol
- d)+35 KJ mol
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
For the hydrolysis of 1 mole of ATP at 37°C. the standard free ent...
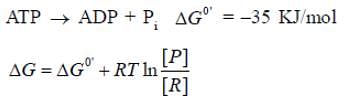


Most Upvoted Answer
For the hydrolysis of 1 mole of ATP at 37°C. the standard free ent...
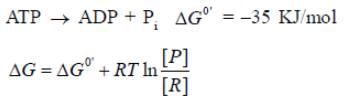


Free Test
FREE
| Start Free Test |
Community Answer
For the hydrolysis of 1 mole of ATP at 37°C. the standard free ent...
°C and pH 7, the standard Gibbs free energy change (∆G°) is -30.5 kJ/mol. This means that the hydrolysis of 1 mole of ATP releases 30.5 kJ of energy.
The hydrolysis of ATP is an exergonic reaction, meaning it releases energy. This energy is used to drive various cellular processes, such as muscle contraction, active transport, and synthesis of macromolecules.
The hydrolysis of ATP involves the breaking of a high-energy phosphate bond, resulting in the formation of ADP (adenosine diphosphate) and inorganic phosphate (Pi). This reaction is catalyzed by the enzyme ATPase.
The released energy from ATP hydrolysis is often used to power cellular processes through the transfer of the phosphate group to other molecules. For example, when ATP donates its phosphate group to glucose, it forms ADP and glucose-6-phosphate, providing energy for the initial steps of glycolysis.
Overall, the hydrolysis of ATP plays a crucial role in cellular energy metabolism, providing the necessary energy for the functioning of various cellular processes.
The hydrolysis of ATP is an exergonic reaction, meaning it releases energy. This energy is used to drive various cellular processes, such as muscle contraction, active transport, and synthesis of macromolecules.
The hydrolysis of ATP involves the breaking of a high-energy phosphate bond, resulting in the formation of ADP (adenosine diphosphate) and inorganic phosphate (Pi). This reaction is catalyzed by the enzyme ATPase.
The released energy from ATP hydrolysis is often used to power cellular processes through the transfer of the phosphate group to other molecules. For example, when ATP donates its phosphate group to glucose, it forms ADP and glucose-6-phosphate, providing energy for the initial steps of glycolysis.
Overall, the hydrolysis of ATP plays a crucial role in cellular energy metabolism, providing the necessary energy for the functioning of various cellular processes.

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer?
Question Description
For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer?.
For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer?.
Solutions for For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For the hydrolysis of 1 mole of ATP at 37°C. the standard free enthalpy change ΔGº= -35 KJ mol-1. Calculate the free enthalpy change ΔG° at the ratio ATP ADP = 100:1(Temperature 37°C. R = 8.3143 J K-1 mol-1. Concentrations of water and inorganic phosphate are to be omitted from the equilibrium equation, assuming that they do not change significantly)a)-46.9 KJ mol-1b)+46.9 KJ mol-1c)-35 KJ mold)+35 KJ molCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















