Defence Exam > Defence Questions > Liquids and gases never showa)diamagnetic pro...
Start Learning for Free
Liquids and gases never show
- a)diamagnetic property
- b)paramagnetic property
- c)ferromagnetic property
- d)electromagnetic property
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Liquids and gases never showa)diamagnetic propertyb)paramagnetic prope...
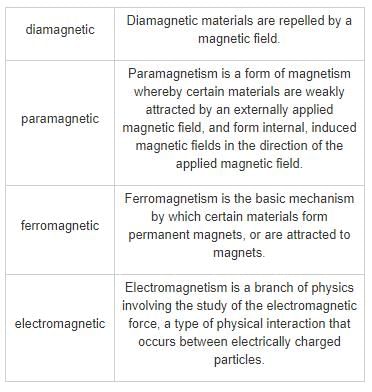
In ferromagnets, the magnetic moments of the individual atoms line and reinforce each other, and that gives the material the large overall magnetic field. The molecules of liquid and gas rearranges every few picoseconds (1012 times per second). Hence, they cannot show ferromagnetic properties.
Most Upvoted Answer
Liquids and gases never showa)diamagnetic propertyb)paramagnetic prope...
Liquids and gases never show ferromagnetic property.
Ferromagnetism is a phenomenon where certain materials exhibit a strong attraction towards a magnetic field and can retain their magnetization even after the external field is removed. This property is commonly observed in solids, specifically in materials such as iron, cobalt, and nickel. However, liquids and gases do not exhibit ferromagnetic behavior. Let's understand why.
1. Definition of ferromagnetism:
Ferromagnetism is the property of certain materials to become permanently magnetized when exposed to a magnetic field. This behavior arises due to the alignment of magnetic moments of individual atoms or ions within the material.
2. Atomic and molecular arrangement:
In solids, the atoms or ions are arranged in a regular lattice structure, allowing for strong magnetic interactions between adjacent atoms. This arrangement facilitates the alignment of atomic magnetic moments, leading to the generation of a macroscopic magnetic field.
3. Randomness in liquids and gases:
In contrast to solids, liquids and gases do not possess a regular arrangement of atoms or ions. The particles in liquids and gases are in constant motion and exhibit a high degree of randomness. This lack of order prevents the establishment of a long-range magnetic order and hinders the alignment of magnetic moments.
4. Weaker magnetic interactions:
The weak intermolecular forces present in liquids and gases result in weaker magnetic interactions between particles. The magnetic moments of individual atoms or molecules in liquids and gases tend to align randomly, causing the cancellation of any net magnetic effect.
5. Temperature effects:
At high temperatures, the thermal energy of particles in liquids and gases is sufficient to disrupt any temporary magnetic alignment that may occur. This thermal agitation prevents the establishment of a long-lasting magnetic order and weakens any magnetic behavior that may be present.
Therefore, due to the lack of regular atomic or molecular arrangement, weaker magnetic interactions, and the influence of thermal energy, liquids and gases do not exhibit ferromagnetic properties.
Ferromagnetism is a phenomenon where certain materials exhibit a strong attraction towards a magnetic field and can retain their magnetization even after the external field is removed. This property is commonly observed in solids, specifically in materials such as iron, cobalt, and nickel. However, liquids and gases do not exhibit ferromagnetic behavior. Let's understand why.
1. Definition of ferromagnetism:
Ferromagnetism is the property of certain materials to become permanently magnetized when exposed to a magnetic field. This behavior arises due to the alignment of magnetic moments of individual atoms or ions within the material.
2. Atomic and molecular arrangement:
In solids, the atoms or ions are arranged in a regular lattice structure, allowing for strong magnetic interactions between adjacent atoms. This arrangement facilitates the alignment of atomic magnetic moments, leading to the generation of a macroscopic magnetic field.
3. Randomness in liquids and gases:
In contrast to solids, liquids and gases do not possess a regular arrangement of atoms or ions. The particles in liquids and gases are in constant motion and exhibit a high degree of randomness. This lack of order prevents the establishment of a long-range magnetic order and hinders the alignment of magnetic moments.
4. Weaker magnetic interactions:
The weak intermolecular forces present in liquids and gases result in weaker magnetic interactions between particles. The magnetic moments of individual atoms or molecules in liquids and gases tend to align randomly, causing the cancellation of any net magnetic effect.
5. Temperature effects:
At high temperatures, the thermal energy of particles in liquids and gases is sufficient to disrupt any temporary magnetic alignment that may occur. This thermal agitation prevents the establishment of a long-lasting magnetic order and weakens any magnetic behavior that may be present.
Therefore, due to the lack of regular atomic or molecular arrangement, weaker magnetic interactions, and the influence of thermal energy, liquids and gases do not exhibit ferromagnetic properties.

|
Explore Courses for Defence exam
|

|
Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer?
Question Description
Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? for Defence 2024 is part of Defence preparation. The Question and answers have been prepared according to the Defence exam syllabus. Information about Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Defence 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer?.
Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? for Defence 2024 is part of Defence preparation. The Question and answers have been prepared according to the Defence exam syllabus. Information about Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Defence 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Defence.
Download more important topics, notes, lectures and mock test series for Defence Exam by signing up for free.
Here you can find the meaning of Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Liquids and gases never showa)diamagnetic propertyb)paramagnetic propertyc)ferromagnetic propertyd)electromagnetic propertyCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Defence tests.

|
Explore Courses for Defence exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















