Class 9 Exam > Class 9 Questions > Diffrence between atoms and molecules . Relat...
Start Learning for Free
Diffrence between atoms and molecules .
?Verified Answer
Diffrence between atoms and molecules . Related: NCERT Solutions - At...
 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
Diffrence between atoms and molecules . Related: NCERT Solutions - At...
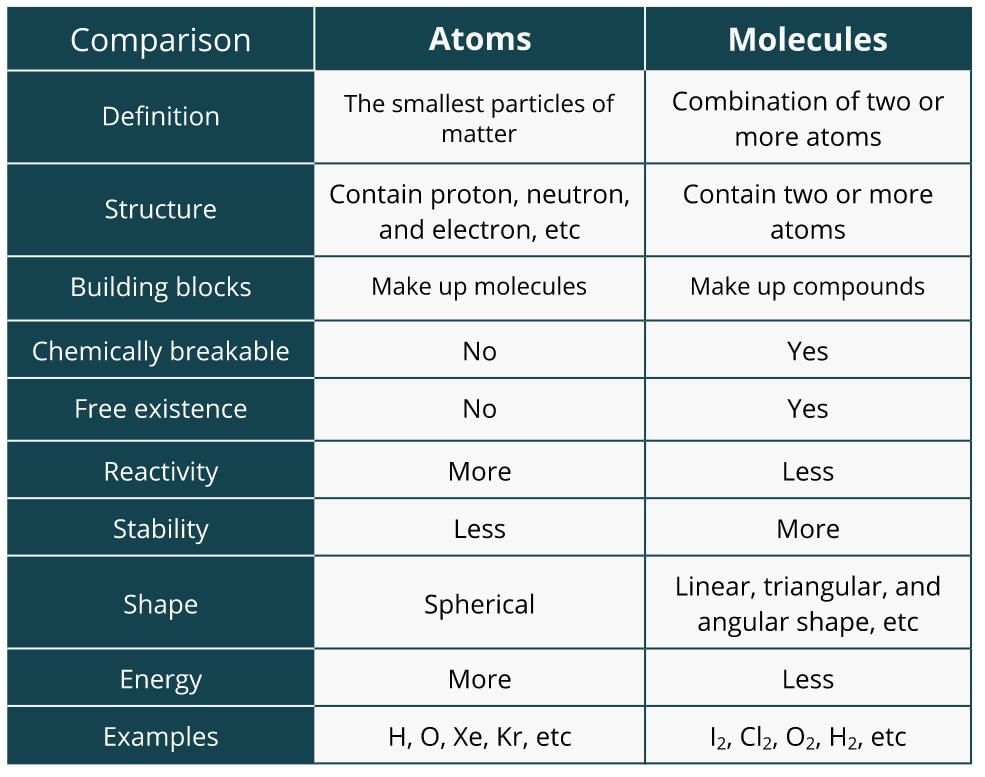
1. Atom is the smallest part of an element while molecules are made of two or More atoms
2. Atoms can't be separated into subatomic particles by chemical reaction whereas molecules can be separated into atoms by chemical reaction.
3. Hydrogen , oxygen , nitrogen are some examples of atoms while H^2O( water), NO^2 are some examples of molecules.
4. Atoms have no bonding whereas molecules have intermolecular forces intra-molecular forces.
5. Atoms may or may not have properties of matter whereas molecules have properties of matter.
2. Atoms can't be separated into subatomic particles by chemical reaction whereas molecules can be separated into atoms by chemical reaction.
3. Hydrogen , oxygen , nitrogen are some examples of atoms while H^2O( water), NO^2 are some examples of molecules.
4. Atoms have no bonding whereas molecules have intermolecular forces intra-molecular forces.
5. Atoms may or may not have properties of matter whereas molecules have properties of matter.
Community Answer
Diffrence between atoms and molecules . Related: NCERT Solutions - At...
Atoms:
- Atoms are the basic building blocks of matter.
- They are the smallest unit of an element that retains its chemical properties.
- Atoms consist of a central nucleus that contains positively charged protons and uncharged neutrons. Surrounding the nucleus are negatively charged electrons.
- Atoms are indivisible and cannot be broken down further without losing their chemical properties.
- Each element is made up of a unique type of atom.
Molecules:
- Molecules are formed when two or more atoms chemically combine.
- They are the smallest unit of a compound that retains its chemical properties.
- Molecules can be made up of atoms of the same element (diatomic molecules) or atoms of different elements (polyatomic molecules).
- In a molecule, atoms are held together by chemical bonds, which can be covalent or ionic.
- Molecules have a distinct set of properties that are different from the properties of individual atoms.
- The properties of a molecule depend on the types and arrangement of atoms within it.
Relationship between Atoms and Molecules:
- Atoms are the building blocks of molecules.
- Molecules are composed of atoms that are chemically bonded together.
- When atoms combine, they form molecules with different properties and characteristics.
- The number and types of atoms present in a molecule determine its chemical formula and properties.
- Molecules can be composed of atoms of the same element (e.g., O2, N2) or different elements (e.g., H2O, CO2).
NCERT Solutions - Atoms And Molecules:
- NCERT Solutions for the chapter "Atoms and Molecules" provide answers to the questions and exercises given in the NCERT textbook for Class 9.
- These solutions help students understand the concepts of atoms, molecules, chemical formulas, and equations.
- The solutions provide step-by-step explanations, diagrams, and examples to facilitate better understanding.
- They cover topics such as the law of conservation of mass, atoms and molecules, writing chemical formulas, balancing chemical equations, and calculating molecular masses.
- NCERT Solutions help students prepare for exams by providing practice questions and solutions for self-assessment.
- They are an important resource for students to clarify their doubts and strengthen their understanding of the subject.
- NCERT Solutions can be accessed through various online platforms, including EduRev, which provides free and reliable study materials for students.
- Atoms are the basic building blocks of matter.
- They are the smallest unit of an element that retains its chemical properties.
- Atoms consist of a central nucleus that contains positively charged protons and uncharged neutrons. Surrounding the nucleus are negatively charged electrons.
- Atoms are indivisible and cannot be broken down further without losing their chemical properties.
- Each element is made up of a unique type of atom.
Molecules:
- Molecules are formed when two or more atoms chemically combine.
- They are the smallest unit of a compound that retains its chemical properties.
- Molecules can be made up of atoms of the same element (diatomic molecules) or atoms of different elements (polyatomic molecules).
- In a molecule, atoms are held together by chemical bonds, which can be covalent or ionic.
- Molecules have a distinct set of properties that are different from the properties of individual atoms.
- The properties of a molecule depend on the types and arrangement of atoms within it.
Relationship between Atoms and Molecules:
- Atoms are the building blocks of molecules.
- Molecules are composed of atoms that are chemically bonded together.
- When atoms combine, they form molecules with different properties and characteristics.
- The number and types of atoms present in a molecule determine its chemical formula and properties.
- Molecules can be composed of atoms of the same element (e.g., O2, N2) or different elements (e.g., H2O, CO2).
NCERT Solutions - Atoms And Molecules:
- NCERT Solutions for the chapter "Atoms and Molecules" provide answers to the questions and exercises given in the NCERT textbook for Class 9.
- These solutions help students understand the concepts of atoms, molecules, chemical formulas, and equations.
- The solutions provide step-by-step explanations, diagrams, and examples to facilitate better understanding.
- They cover topics such as the law of conservation of mass, atoms and molecules, writing chemical formulas, balancing chemical equations, and calculating molecular masses.
- NCERT Solutions help students prepare for exams by providing practice questions and solutions for self-assessment.
- They are an important resource for students to clarify their doubts and strengthen their understanding of the subject.
- NCERT Solutions can be accessed through various online platforms, including EduRev, which provides free and reliable study materials for students.
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules?
Question Description
Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules?.
Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules?.
Solutions for Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? defined & explained in the simplest way possible. Besides giving the explanation of
Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules?, a detailed solution for Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? has been provided alongside types of Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? theory, EduRev gives you an
ample number of questions to practice Diffrence between atoms and molecules . Related: NCERT Solutions - Atoms And Molecules? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Test: The Fundamental Unit of Life- Case Based Type Questions- 1
Test | 10 questions
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























