Class 9 Exam > Class 9 Questions > What is the difference between evaporation an...
Start Learning for Free
What is the difference between evaporation and boiling?
Verified Answer
What is the difference between evaporation and boiling?
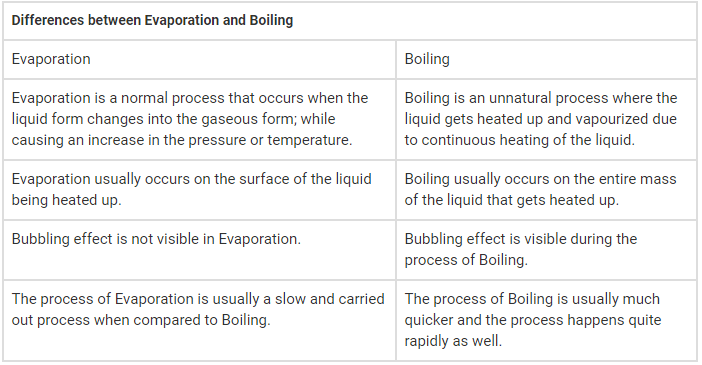
 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
What is the difference between evaporation and boiling?
Evaporation 1=Evaporation occurs even below boiling point temperature. 2=It occurs only at the surface. 3=Evaporation causes cooling. Boiling. 1=Boiling occurs at particular temperature. 2=It occurs in the bulk of liquid. 3=It does not causes cooling
Community Answer
What is the difference between evaporation and boiling?
Evaporation vs. Boiling
Evaporation and boiling are both processes that involve a liquid turning into a gas, but they differ in several key ways.
Evaporation:
- Evaporation is a slow process that occurs at the surface of a liquid.
- It takes place at temperatures below the boiling point of the liquid.
- The molecules at the surface of the liquid gain enough energy to escape into the air as vapor.
- Evaporation can happen at any temperature, even at room temperature, and does not require the entire liquid to reach a specific temperature.
- It is a spontaneous process driven by the heat energy from the surroundings.
- Evaporation leads to the cooling of the remaining liquid as the molecules with higher kinetic energy leave the surface.
Boiling:
- Boiling is a rapid process that occurs throughout the liquid.
- It happens at a specific temperature called the boiling point of the liquid.
- The entire liquid reaches the boiling point, and bubbles of vapor form within the liquid and rise to the surface.
- Boiling requires a constant source of heat to maintain the temperature at or above the boiling point.
- It is a non-spontaneous process that requires an external heat source to provide the necessary energy for the liquid to vaporize.
- Boiling does not lead to the cooling of the liquid, as the heat input is continuously required to keep the liquid at the boiling point.
In conclusion, while both evaporation and boiling involve the conversion of a liquid into a gas, they differ in terms of speed, temperature requirements, energy source, and impact on the remaining liquid.
Evaporation and boiling are both processes that involve a liquid turning into a gas, but they differ in several key ways.
Evaporation:
- Evaporation is a slow process that occurs at the surface of a liquid.
- It takes place at temperatures below the boiling point of the liquid.
- The molecules at the surface of the liquid gain enough energy to escape into the air as vapor.
- Evaporation can happen at any temperature, even at room temperature, and does not require the entire liquid to reach a specific temperature.
- It is a spontaneous process driven by the heat energy from the surroundings.
- Evaporation leads to the cooling of the remaining liquid as the molecules with higher kinetic energy leave the surface.
Boiling:
- Boiling is a rapid process that occurs throughout the liquid.
- It happens at a specific temperature called the boiling point of the liquid.
- The entire liquid reaches the boiling point, and bubbles of vapor form within the liquid and rise to the surface.
- Boiling requires a constant source of heat to maintain the temperature at or above the boiling point.
- It is a non-spontaneous process that requires an external heat source to provide the necessary energy for the liquid to vaporize.
- Boiling does not lead to the cooling of the liquid, as the heat input is continuously required to keep the liquid at the boiling point.
In conclusion, while both evaporation and boiling involve the conversion of a liquid into a gas, they differ in terms of speed, temperature requirements, energy source, and impact on the remaining liquid.
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
What is the difference between evaporation and boiling?
Question Description
What is the difference between evaporation and boiling? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about What is the difference between evaporation and boiling? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the difference between evaporation and boiling?.
What is the difference between evaporation and boiling? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about What is the difference between evaporation and boiling? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the difference between evaporation and boiling?.
Solutions for What is the difference between evaporation and boiling? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of What is the difference between evaporation and boiling? defined & explained in the simplest way possible. Besides giving the explanation of
What is the difference between evaporation and boiling?, a detailed solution for What is the difference between evaporation and boiling? has been provided alongside types of What is the difference between evaporation and boiling? theory, EduRev gives you an
ample number of questions to practice What is the difference between evaporation and boiling? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.




















