Class 11 Exam > Class 11 Questions > What is Chichibabin reaction's mechanism?
Start Learning for Free
What is Chichibabin reaction's mechanism?
Most Upvoted Answer
What is Chichibabin reaction's mechanism?
The Chichibabin reaction is a method for producing 2-aminopyridine derivatives by the reaction of pyridine with sodium amide. It was reported by Aleksei Chichibabin in 1914. The following is the overall form of the general reaction:
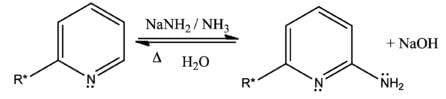
The direct amination of pyridine with sodium amide takes place in liquid ammonia. Following the addition elimination mechanism first a nucleophilic NH2− is added while a hydride (H−) is leaving.
Ciganek describes an example of an intramolecular Chichibabin reaction in which a nitrile group on a fused ring is the source of nitrogen in amination.Community Answer
What is Chichibabin reaction's mechanism?
Chichibabin reaction mechanism
The Chichibabin reaction is a chemical reaction that involves the formation of pyridine derivatives from the reaction of a nitrogen-containing compound with a strong base and an electrophile. It was discovered by Russian chemist Aleksandr Chichibabin in the early 20th century.
The mechanism of the Chichibabin reaction can be divided into several steps:
Step 1: Deprotonation
The reaction begins with the deprotonation of the nitrogen-containing compound by a strong base. The base abstracts a proton from the nitrogen atom, resulting in the formation of a negatively charged nitrogen species called a nitrogen anion. This deprotonation step is often the rate-determining step of the reaction.
Step 2: Formation of a carbanion
The nitrogen anion formed in the first step acts as a nucleophile and attacks the electrophilic carbon atom of the electrophile, which is typically an alkyl halide or an acyl chloride. This leads to the formation of a carbanion intermediate.
Step 3: Rearrangement
The carbanion intermediate undergoes a rearrangement through a series of bond shifts, resulting in the formation of a new carbon-nitrogen bond. This rearrangement is usually facilitated by the presence of electron-withdrawing groups on the nitrogen atom or neighboring carbon atoms.
Step 4: Protonation
In the final step, the newly formed pyridine derivative is protonated by a proton source, such as an acid or water. This protonation step restores the aromaticity of the pyridine ring and gives the final product of the Chichibabin reaction.
Overall reaction:
The overall reaction can be represented as follows:
Nitrogen-containing compound + Electrophile → Pyridine derivative
The Chichibabin reaction is a valuable synthetic tool for the synthesis of pyridine derivatives, which are important building blocks in the pharmaceutical and agrochemical industries. The reaction can be applied to a wide range of nitrogen-containing compounds, providing access to diverse pyridine structures.
Conclusion:
The Chichibabin reaction proceeds through a series of steps, including deprotonation, formation of a carbanion intermediate, rearrangement, and protonation. Understanding the mechanism of this reaction allows chemists to design and optimize synthetic routes for the preparation of pyridine derivatives.
The Chichibabin reaction is a chemical reaction that involves the formation of pyridine derivatives from the reaction of a nitrogen-containing compound with a strong base and an electrophile. It was discovered by Russian chemist Aleksandr Chichibabin in the early 20th century.
The mechanism of the Chichibabin reaction can be divided into several steps:
Step 1: Deprotonation
The reaction begins with the deprotonation of the nitrogen-containing compound by a strong base. The base abstracts a proton from the nitrogen atom, resulting in the formation of a negatively charged nitrogen species called a nitrogen anion. This deprotonation step is often the rate-determining step of the reaction.
Step 2: Formation of a carbanion
The nitrogen anion formed in the first step acts as a nucleophile and attacks the electrophilic carbon atom of the electrophile, which is typically an alkyl halide or an acyl chloride. This leads to the formation of a carbanion intermediate.
Step 3: Rearrangement
The carbanion intermediate undergoes a rearrangement through a series of bond shifts, resulting in the formation of a new carbon-nitrogen bond. This rearrangement is usually facilitated by the presence of electron-withdrawing groups on the nitrogen atom or neighboring carbon atoms.
Step 4: Protonation
In the final step, the newly formed pyridine derivative is protonated by a proton source, such as an acid or water. This protonation step restores the aromaticity of the pyridine ring and gives the final product of the Chichibabin reaction.
Overall reaction:
The overall reaction can be represented as follows:
Nitrogen-containing compound + Electrophile → Pyridine derivative
The Chichibabin reaction is a valuable synthetic tool for the synthesis of pyridine derivatives, which are important building blocks in the pharmaceutical and agrochemical industries. The reaction can be applied to a wide range of nitrogen-containing compounds, providing access to diverse pyridine structures.
Conclusion:
The Chichibabin reaction proceeds through a series of steps, including deprotonation, formation of a carbanion intermediate, rearrangement, and protonation. Understanding the mechanism of this reaction allows chemists to design and optimize synthetic routes for the preparation of pyridine derivatives.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
What is Chichibabin reaction's mechanism?
Question Description
What is Chichibabin reaction's mechanism? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about What is Chichibabin reaction's mechanism? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is Chichibabin reaction's mechanism?.
What is Chichibabin reaction's mechanism? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about What is Chichibabin reaction's mechanism? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is Chichibabin reaction's mechanism?.
Solutions for What is Chichibabin reaction's mechanism? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of What is Chichibabin reaction's mechanism? defined & explained in the simplest way possible. Besides giving the explanation of
What is Chichibabin reaction's mechanism?, a detailed solution for What is Chichibabin reaction's mechanism? has been provided alongside types of What is Chichibabin reaction's mechanism? theory, EduRev gives you an
ample number of questions to practice What is Chichibabin reaction's mechanism? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























