IIT JAM Exam > IIT JAM Questions > Ultraviolet light of wavelength 350 nmand int...
Start Learning for Free
Ultraviolet light of wavelength 350 nm and intensity 1.00 W/m2 is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s, 1 eV = 1.6 x 10-19 J)
Correct answer is between '8.7,8.9'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is direct...
Energy of a photons is
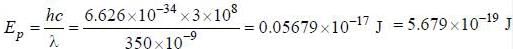
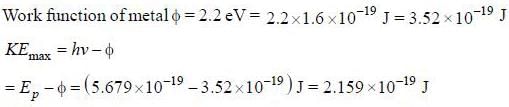
Number of photons passing through unit surface per second is
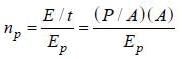
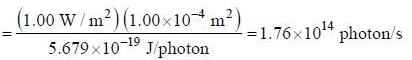
The rate at which photoelectrons are emitted
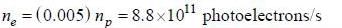
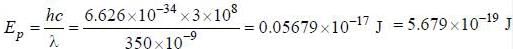
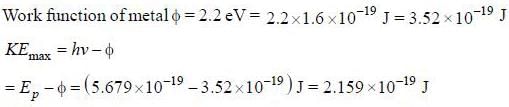
Number of photons passing through unit surface per second is
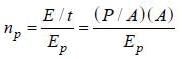
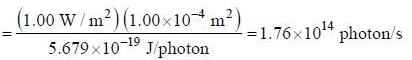
The rate at which photoelectrons are emitted
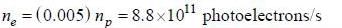
Most Upvoted Answer
Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is direct...
Given data:
Wavelength of ultraviolet light, λ = 350 nm = 350 × 10⁻⁹ m
Intensity of light, I = 1.00 W/m²
Work function of metal surface, φ = 2.2 eV = 2.2 × 1.6 × 10⁻¹⁹ J
Percentage of photons producing photoelectrons, n = 0.50%
Area of metal surface, A = 1.00 cm² = 1.00 × 10⁻⁴ m²
Planck’s constant, h = 6.626 × 10⁻³⁴ J s
Speed of light, c = 3 × 10⁸ m/s
To find: Number of photoelectrons emitted per second
Solution:
1. Energy of photons:
The energy of a photon is given by the equation:
E = hc/λ
where h is Planck’s constant and c is the speed of light.
Substituting the given values, we get:
E = (6.626 × 10⁻³⁴ J s) × (3 × 10⁸ m/s) / (350 × 10⁻⁹ m)
E = 5.66 × 10⁻¹⁹ J
2. Threshold frequency:
The threshold frequency f₀ is the minimum frequency of light required to eject an electron from the metal surface. It is related to the work function φ by the equation:
φ = hf₀
where f₀ is the threshold frequency.
Substituting the given values, we get:
f₀ = φ/h
f₀ = (2.2 × 1.6 × 10⁻¹⁹ J) / (6.626 × 10⁻³⁴ J s)
f₀ = 5.11 × 10¹⁴ Hz
3. Number of photons:
The intensity of light is given by the equation:
I = nhf
where n is the number of photons per unit area per unit time, h is Planck’s constant, and f is the frequency of the light.
Rearranging the equation, we get:
n = I / hf
Substituting the given values, we get:
n = (1.00 W/m²) / [(6.626 × 10⁻³⁴ J s) × (3 × 10⁸ m/s) / (350 × 10⁻⁹ m)]
n = 5.70 × 10¹⁵ photons/m²s
4. Number of photoelectrons:
The number of photoelectrons emitted per unit time is given by the equation:
I₀ = nAq
where I₀ is the current due to photoelectrons, q is the charge of an electron, and A is the area of the metal surface.
The number of photoelectrons emitted per second is equal to I₀/q.
Substituting the given values, we get:
I₀ = nAq = (5.70 × 10¹⁵ photons/m²s) × (1.00 × 10⁻⁴ m²) × (0.50%) × (1.6 × 10⁻¹⁹ C/photon)
I₀ = 4.59 × 10⁻¹² A
Wavelength of ultraviolet light, λ = 350 nm = 350 × 10⁻⁹ m
Intensity of light, I = 1.00 W/m²
Work function of metal surface, φ = 2.2 eV = 2.2 × 1.6 × 10⁻¹⁹ J
Percentage of photons producing photoelectrons, n = 0.50%
Area of metal surface, A = 1.00 cm² = 1.00 × 10⁻⁴ m²
Planck’s constant, h = 6.626 × 10⁻³⁴ J s
Speed of light, c = 3 × 10⁸ m/s
To find: Number of photoelectrons emitted per second
Solution:
1. Energy of photons:
The energy of a photon is given by the equation:
E = hc/λ
where h is Planck’s constant and c is the speed of light.
Substituting the given values, we get:
E = (6.626 × 10⁻³⁴ J s) × (3 × 10⁸ m/s) / (350 × 10⁻⁹ m)
E = 5.66 × 10⁻¹⁹ J
2. Threshold frequency:
The threshold frequency f₀ is the minimum frequency of light required to eject an electron from the metal surface. It is related to the work function φ by the equation:
φ = hf₀
where f₀ is the threshold frequency.
Substituting the given values, we get:
f₀ = φ/h
f₀ = (2.2 × 1.6 × 10⁻¹⁹ J) / (6.626 × 10⁻³⁴ J s)
f₀ = 5.11 × 10¹⁴ Hz
3. Number of photons:
The intensity of light is given by the equation:
I = nhf
where n is the number of photons per unit area per unit time, h is Planck’s constant, and f is the frequency of the light.
Rearranging the equation, we get:
n = I / hf
Substituting the given values, we get:
n = (1.00 W/m²) / [(6.626 × 10⁻³⁴ J s) × (3 × 10⁸ m/s) / (350 × 10⁻⁹ m)]
n = 5.70 × 10¹⁵ photons/m²s
4. Number of photoelectrons:
The number of photoelectrons emitted per unit time is given by the equation:
I₀ = nAq
where I₀ is the current due to photoelectrons, q is the charge of an electron, and A is the area of the metal surface.
The number of photoelectrons emitted per second is equal to I₀/q.
Substituting the given values, we get:
I₀ = nAq = (5.70 × 10¹⁵ photons/m²s) × (1.00 × 10⁻⁴ m²) × (0.50%) × (1.6 × 10⁻¹⁹ C/photon)
I₀ = 4.59 × 10⁻¹² A

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer?
Question Description
Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer?.
Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer?.
Solutions for Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer?, a detailed solution for Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? has been provided alongside types of Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Ultraviolet light of wavelength 350 nmand intensity 1.00 W/m2is directed at a metal surface of work function 2.2 eV. If 0.50% of the incident photons produce photoelectrons, the number of photo electrons emitted per second, if the metal surface lias an area of 1.00 cm2, is equal to ____ x 1011 photoelectrons/s. (upto one decimal place) (h = 6.626 x 10-34 J s, c = 3 x 108 m/s,1 eV = 1.6 x 10-19 J)Correct answer is between '8.7,8.9'. Can you explain this answer? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















