Class 12 Exam > Class 12 Questions > What is wurtz reaction?explain?
Start Learning for Free
What is wurtz reaction?explain?
Most Upvoted Answer
What is wurtz reaction?explain?
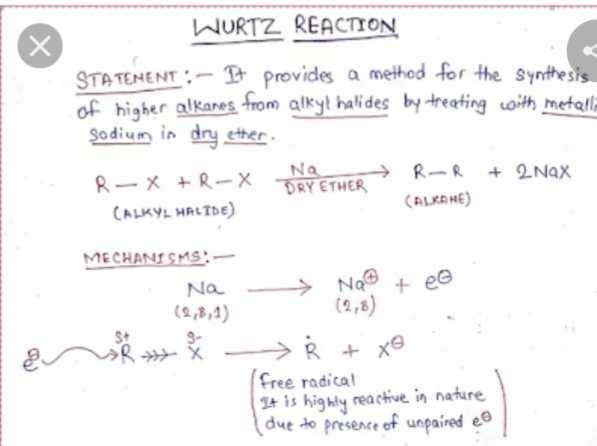
Community Answer
What is wurtz reaction?explain?
Wurtz Reaction:
The Wurtz reaction is a chemical reaction that involves the coupling of two alkyl halides to form a higher alkane. It was discovered by Charles-Adolphe Wurtz in 1855 and has since become an important tool in organic synthesis. The reaction is typically carried out in the presence of a strong base, such as sodium or potassium, which acts as a catalyst.
Reaction Mechanism:
The Wurtz reaction proceeds through a free radical mechanism. Here is the step-by-step mechanism:
1. Homolytic cleavage: The reaction begins with the homolytic cleavage of the alkyl halides, which results in the formation of alkyl radicals. This process is facilitated by the presence of the strong base.
2. Radical combination: The alkyl radicals then combine to form a higher alkane. This step involves the formation of a new carbon-carbon bond.
3. Formation of byproducts: In some cases, side reactions can occur, leading to the formation of byproducts. For example, if the alkyl halides contain more than one halogen atom, halogen exchange reactions may take place.
Scope and Limitations:
The Wurtz reaction is most commonly used for the synthesis of symmetrical alkanes, where both alkyl groups are identical. It is particularly useful for the synthesis of higher alkanes, as it allows for the coupling of multiple alkyl halides. However, the reaction is not suitable for the synthesis of unsymmetrical alkanes, as it results in a mixture of products.
Examples:
1. Formation of ethane: When two molecules of methyl iodide (CH3I) react with sodium metal, the Wurtz reaction occurs, leading to the formation of ethane (CH3-CH3).
2. Formation of propane: When one molecule of ethyl bromide (CH3CH2Br) and one molecule of methyl iodide (CH3I) react with sodium metal, the Wurtz reaction occurs, leading to the formation of propane (CH3CH2CH3).
3. Formation of butane: When two molecules of ethyl bromide (CH3CH2Br) react with sodium metal, the Wurtz reaction occurs, leading to the formation of butane (CH3CH2CH2CH3).
Conclusion:
The Wurtz reaction is a valuable tool in organic synthesis for the formation of higher alkanes. It allows for the coupling of alkyl halides to form symmetrical alkanes. However, it is important to note that the reaction is limited to the synthesis of symmetrical alkanes and may result in the formation of byproducts.
The Wurtz reaction is a chemical reaction that involves the coupling of two alkyl halides to form a higher alkane. It was discovered by Charles-Adolphe Wurtz in 1855 and has since become an important tool in organic synthesis. The reaction is typically carried out in the presence of a strong base, such as sodium or potassium, which acts as a catalyst.
Reaction Mechanism:
The Wurtz reaction proceeds through a free radical mechanism. Here is the step-by-step mechanism:
1. Homolytic cleavage: The reaction begins with the homolytic cleavage of the alkyl halides, which results in the formation of alkyl radicals. This process is facilitated by the presence of the strong base.
2. Radical combination: The alkyl radicals then combine to form a higher alkane. This step involves the formation of a new carbon-carbon bond.
3. Formation of byproducts: In some cases, side reactions can occur, leading to the formation of byproducts. For example, if the alkyl halides contain more than one halogen atom, halogen exchange reactions may take place.
Scope and Limitations:
The Wurtz reaction is most commonly used for the synthesis of symmetrical alkanes, where both alkyl groups are identical. It is particularly useful for the synthesis of higher alkanes, as it allows for the coupling of multiple alkyl halides. However, the reaction is not suitable for the synthesis of unsymmetrical alkanes, as it results in a mixture of products.
Examples:
1. Formation of ethane: When two molecules of methyl iodide (CH3I) react with sodium metal, the Wurtz reaction occurs, leading to the formation of ethane (CH3-CH3).
2. Formation of propane: When one molecule of ethyl bromide (CH3CH2Br) and one molecule of methyl iodide (CH3I) react with sodium metal, the Wurtz reaction occurs, leading to the formation of propane (CH3CH2CH3).
3. Formation of butane: When two molecules of ethyl bromide (CH3CH2Br) react with sodium metal, the Wurtz reaction occurs, leading to the formation of butane (CH3CH2CH2CH3).
Conclusion:
The Wurtz reaction is a valuable tool in organic synthesis for the formation of higher alkanes. It allows for the coupling of alkyl halides to form symmetrical alkanes. However, it is important to note that the reaction is limited to the synthesis of symmetrical alkanes and may result in the formation of byproducts.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
What is wurtz reaction?explain?
Question Description
What is wurtz reaction?explain? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What is wurtz reaction?explain? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is wurtz reaction?explain?.
What is wurtz reaction?explain? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What is wurtz reaction?explain? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is wurtz reaction?explain?.
Solutions for What is wurtz reaction?explain? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of What is wurtz reaction?explain? defined & explained in the simplest way possible. Besides giving the explanation of
What is wurtz reaction?explain?, a detailed solution for What is wurtz reaction?explain? has been provided alongside types of What is wurtz reaction?explain? theory, EduRev gives you an
ample number of questions to practice What is wurtz reaction?explain? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















