JEE Exam > JEE Questions > Among the species given below, the total numb...
Start Learning for Free
Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2- (superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4
Correct answer is '1'. Can you explain this answer?
Verified Answer
Among the species given below, the total number of diamagnetic species...
Hydrogen atom is paramagnetic because it has one electron.
The given species NO2 monomer is paramagnetic because it has an odd electron.
The given species O2− superoxide is paramagnetic because it has one unpaired electron in its π*.
The given species S2 is paramagnetic because it has two unpaired electrons in its π*.
The given species Mn3O4 is paramagnetic because it exists as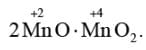
In the given species (NH4)2 [FeCl4], iron exists in +2
oxidation state. Thus, it is paramagnetic.
In the given species (NH4)2 [NiCl4], nickel exists in +2
oxidation state. Thus, it is paramagnetic.
In the given species K2MnO4, manganese exists in +6 oxidation state. Thus, it is paramagnetic.
In the given species K2CrO4, manganese exists in +6
oxidation state. Thus, it is diamagnetic.
The given species NO2 monomer is paramagnetic because it has an odd electron.
The given species O2− superoxide is paramagnetic because it has one unpaired electron in its π*.
The given species S2 is paramagnetic because it has two unpaired electrons in its π*.
The given species Mn3O4 is paramagnetic because it exists as
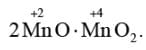
In the given species (NH4)2 [FeCl4], iron exists in +2
oxidation state. Thus, it is paramagnetic.
In the given species (NH4)2 [NiCl4], nickel exists in +2
oxidation state. Thus, it is paramagnetic.
In the given species K2MnO4, manganese exists in +6 oxidation state. Thus, it is paramagnetic.
In the given species K2CrO4, manganese exists in +6
oxidation state. Thus, it is diamagnetic.
Most Upvoted Answer
Among the species given below, the total number of diamagnetic species...
Total Number of Diamagnetic Species
Diamagnetic species are those which have all electrons paired up and do not possess any unpaired electrons. Let us analyze each species given in the question to find out the number of diamagnetic species.
1. H atom - Diamagnetic
The hydrogen atom has only one electron which is paired up with the proton in the nucleus, making it diamagnetic.
2. NO2 monomer - Paramagnetic
NO2 has an unpaired electron and is paramagnetic.
3. O2- (superoxide) - Paramagnetic
O2- has one unpaired electron, making it paramagnetic.
4. Dimeric sulfur in vapour phase - Paramagnetic
Dimeric sulfur has unpaired electrons, making it paramagnetic.
5. Mn3O4 - Paramagnetic
Mn3O4 contains Mn2+ and Mn3+ ions, both of which have unpaired electrons, making it paramagnetic.
6. (NH4)2[FeCl4] - Paramagnetic
FeCl4 has unpaired electrons, making it paramagnetic.
7. (NH4)2[NiCl4] - Paramagnetic
NiCl4 has unpaired electrons, making it paramagnetic.
8. K2MnO4 - Paramagnetic
MnO4 has unpaired electrons, making it paramagnetic.
9. K2CrO4 - Paramagnetic
CrO4 has unpaired electrons, making it paramagnetic.
Therefore, out of the given species, only H atom is diamagnetic, making the total number of diamagnetic species 1.
Diamagnetic species are those which have all electrons paired up and do not possess any unpaired electrons. Let us analyze each species given in the question to find out the number of diamagnetic species.
1. H atom - Diamagnetic
The hydrogen atom has only one electron which is paired up with the proton in the nucleus, making it diamagnetic.
2. NO2 monomer - Paramagnetic
NO2 has an unpaired electron and is paramagnetic.
3. O2- (superoxide) - Paramagnetic
O2- has one unpaired electron, making it paramagnetic.
4. Dimeric sulfur in vapour phase - Paramagnetic
Dimeric sulfur has unpaired electrons, making it paramagnetic.
5. Mn3O4 - Paramagnetic
Mn3O4 contains Mn2+ and Mn3+ ions, both of which have unpaired electrons, making it paramagnetic.
6. (NH4)2[FeCl4] - Paramagnetic
FeCl4 has unpaired electrons, making it paramagnetic.
7. (NH4)2[NiCl4] - Paramagnetic
NiCl4 has unpaired electrons, making it paramagnetic.
8. K2MnO4 - Paramagnetic
MnO4 has unpaired electrons, making it paramagnetic.
9. K2CrO4 - Paramagnetic
CrO4 has unpaired electrons, making it paramagnetic.
Therefore, out of the given species, only H atom is diamagnetic, making the total number of diamagnetic species 1.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer?
Question Description
Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer?.
Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer?.
Solutions for Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer?, a detailed solution for Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? has been provided alongside types of Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Among the species given below, the total number of diamagnetic species is ______. H atom, NO2 monomer, O2-(superoxide), dimeric sulphur in vapour phase, Mn3O4, (NH4)2[FeCl4], (NH4)2[NiCl4], K2MnO4, K2CrO4Correct answer is '1'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























