Defence Exam > Defence Questions > Which one of the following is the most charac...
Start Learning for Free
Which one of the following is the most characteristic property of an element?
- a)Density
- b)Boiling point
- c)Mass number
- d)Atomic number
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which one of the following is the most characteristic property of an e...
Atomic number is the most characteristic property of an element.
Most Upvoted Answer
Which one of the following is the most characteristic property of an e...
Atomic number: The most characteristic property of an element
Introduction:
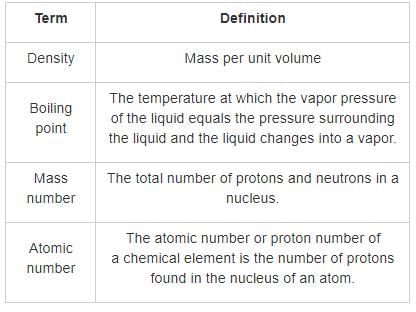
The characteristic properties of an element are the unique qualities that distinguish it from other elements. These properties are essential for identifying and categorizing elements in the periodic table. Among the given options, the most characteristic property of an element is its atomic number.
Explanation:
1. Atomic number:
- The atomic number of an element is the number of protons present in the nucleus of its atom.
- It is denoted by the symbol 'Z' and is specific to each element.
- The atomic number determines the element's position in the periodic table and its chemical identity.
- For example, hydrogen has an atomic number of 1, which means it has one proton in its nucleus.
2. Density:
- Density is a physical property of matter that describes the mass of a substance per unit volume.
- While density can vary among different elements, it is not a unique characteristic property.
- Elements can have similar densities, and density can also be influenced by factors such as temperature and pressure.
3. Boiling point:
- The boiling point of an element is the temperature at which it changes from a liquid to a gas.
- Boiling points can vary significantly among elements, and they are influenced by various factors such as intermolecular forces and atomic structure.
- While boiling point can provide some information about an element, it is not a unique characteristic property.
4. Mass number:
- The mass number of an element is the sum of its protons and neutrons.
- It is denoted by the symbol 'A' and can vary among different isotopes of the same element.
- Mass number alone does not provide enough information to identify or categorize an element uniquely.
Conclusion:
Among the given options, the most characteristic property of an element is its atomic number. The atomic number determines an element's position in the periodic table, its chemical identity, and the number of protons in its nucleus. While other properties such as density, boiling point, and mass number can provide useful information, they are not as specific and unique as the atomic number.
Introduction:
The characteristic properties of an element are the unique qualities that distinguish it from other elements. These properties are essential for identifying and categorizing elements in the periodic table. Among the given options, the most characteristic property of an element is its atomic number.
Explanation:
1. Atomic number:
- The atomic number of an element is the number of protons present in the nucleus of its atom.
- It is denoted by the symbol 'Z' and is specific to each element.
- The atomic number determines the element's position in the periodic table and its chemical identity.
- For example, hydrogen has an atomic number of 1, which means it has one proton in its nucleus.
2. Density:
- Density is a physical property of matter that describes the mass of a substance per unit volume.
- While density can vary among different elements, it is not a unique characteristic property.
- Elements can have similar densities, and density can also be influenced by factors such as temperature and pressure.
3. Boiling point:
- The boiling point of an element is the temperature at which it changes from a liquid to a gas.
- Boiling points can vary significantly among elements, and they are influenced by various factors such as intermolecular forces and atomic structure.
- While boiling point can provide some information about an element, it is not a unique characteristic property.
4. Mass number:
- The mass number of an element is the sum of its protons and neutrons.
- It is denoted by the symbol 'A' and can vary among different isotopes of the same element.
- Mass number alone does not provide enough information to identify or categorize an element uniquely.
Conclusion:
Among the given options, the most characteristic property of an element is its atomic number. The atomic number determines an element's position in the periodic table, its chemical identity, and the number of protons in its nucleus. While other properties such as density, boiling point, and mass number can provide useful information, they are not as specific and unique as the atomic number.

|
Explore Courses for Defence exam
|

|
Similar Defence Doubts
Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer?
Question Description
Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? for Defence 2024 is part of Defence preparation. The Question and answers have been prepared according to the Defence exam syllabus. Information about Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Defence 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer?.
Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? for Defence 2024 is part of Defence preparation. The Question and answers have been prepared according to the Defence exam syllabus. Information about Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Defence 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Defence.
Download more important topics, notes, lectures and mock test series for Defence Exam by signing up for free.
Here you can find the meaning of Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following is the most characteristic property of an element?a)Densityb)Boiling pointc)Mass numberd)Atomic numberCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Defence tests.

|
Explore Courses for Defence exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















