Class 12 Exam > Class 12 Questions > What is the principle behind the zone refinin...
Start Learning for Free
What is the principle behind the zone refining?
Most Upvoted Answer
What is the principle behind the zone refining?
Principle behind Zone Refining:
Zone refining is a process used to purify metals, such as germanium, silicon, and other metals. It was first introduced by William Pfann in 1952. The principle behind zone refining is based on the fact that impurities have different melting points than the main metal.
Process:
Zone refining involves the following steps:
1. A rod of the impure metal is taken and then heated at one end with the help of a heater.
2. The temperature of the heater is higher than the melting point of the impurities but lower than the melting point of the metal.
3. As a result, a molten zone is formed between the heated and cooled ends of the rod.
4. The impurities in the molten zone get concentrated in it, and the pure metal gets transferred to the colder end of the rod.
5. The molten zone is then moved in a controlled manner along the rod.
6. The impurities keep getting concentrated in the molten zone, and the pure metal keeps getting transferred to the colder end.
7. This process is continued until the entire rod gets purified.
Advantages:
1. It is a simple and effective method for obtaining highly pure metals.
2. It can be used for batch processing of metals.
3. It can efficiently remove impurities from the metal.
Limitations:
1. The process is slow and time-consuming.
2. It can only be used for metals with a low melting point.
3. The process is expensive due to the need for specialized equipment.
Conclusion:
Zone refining is a significant process for the purification of metals. It is a simple and effective method that can remove impurities from the metal. However, it is a slow and time-consuming process and requires specialized equipment.
Zone refining is a process used to purify metals, such as germanium, silicon, and other metals. It was first introduced by William Pfann in 1952. The principle behind zone refining is based on the fact that impurities have different melting points than the main metal.
Process:
Zone refining involves the following steps:
1. A rod of the impure metal is taken and then heated at one end with the help of a heater.
2. The temperature of the heater is higher than the melting point of the impurities but lower than the melting point of the metal.
3. As a result, a molten zone is formed between the heated and cooled ends of the rod.
4. The impurities in the molten zone get concentrated in it, and the pure metal gets transferred to the colder end of the rod.
5. The molten zone is then moved in a controlled manner along the rod.
6. The impurities keep getting concentrated in the molten zone, and the pure metal keeps getting transferred to the colder end.
7. This process is continued until the entire rod gets purified.
Advantages:
1. It is a simple and effective method for obtaining highly pure metals.
2. It can be used for batch processing of metals.
3. It can efficiently remove impurities from the metal.
Limitations:
1. The process is slow and time-consuming.
2. It can only be used for metals with a low melting point.
3. The process is expensive due to the need for specialized equipment.
Conclusion:
Zone refining is a significant process for the purification of metals. It is a simple and effective method that can remove impurities from the metal. However, it is a slow and time-consuming process and requires specialized equipment.
Community Answer
What is the principle behind the zone refining?
The principle of zone refining is that the impurities in an ore of metal are more soluble in the melt state when compared to the corresponding solid state of the impurities. It is based upon fractional crystallization.
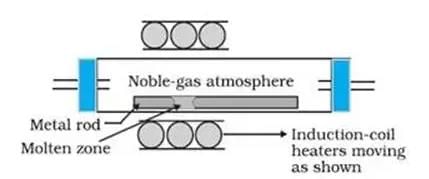
In the zone refining process, a circular mobile heater is fixed at one end of the metal rod which is made up of the impure metal. Now, the circular mobile heater is moved slowly across the metal rod. The metallic impurities melt at the temporary position of this heater. The melt containing the impurities moves forward along with the heater through the entirety of the metal rod. The pure metal is left to solidify as the heater moves along the rod. As the heater moves forward, the concentration of the impurities in the melt increases and these impurities are accumulated at one end of the metal rod.
The process described above is repeated many times in the same direction. The end of the rod in which the impurities have now accumulated in is cut off, leaving behind the pure metal.
Zone refining is a very useful method to get metals with very high purity such as silicon and germanium.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
What is the principle behind the zone refining?
Question Description
What is the principle behind the zone refining? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What is the principle behind the zone refining? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the principle behind the zone refining?.
What is the principle behind the zone refining? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What is the principle behind the zone refining? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the principle behind the zone refining?.
Solutions for What is the principle behind the zone refining? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of What is the principle behind the zone refining? defined & explained in the simplest way possible. Besides giving the explanation of
What is the principle behind the zone refining?, a detailed solution for What is the principle behind the zone refining? has been provided alongside types of What is the principle behind the zone refining? theory, EduRev gives you an
ample number of questions to practice What is the principle behind the zone refining? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















