Class 12 Exam > Class 12 Questions > Concept map for p-block elements ( group 13 )...
Start Learning for Free
Concept map for p-block elements ( group 13 ) ?
Most Upvoted Answer
Concept map for p-block elements ( group 13 ) ?
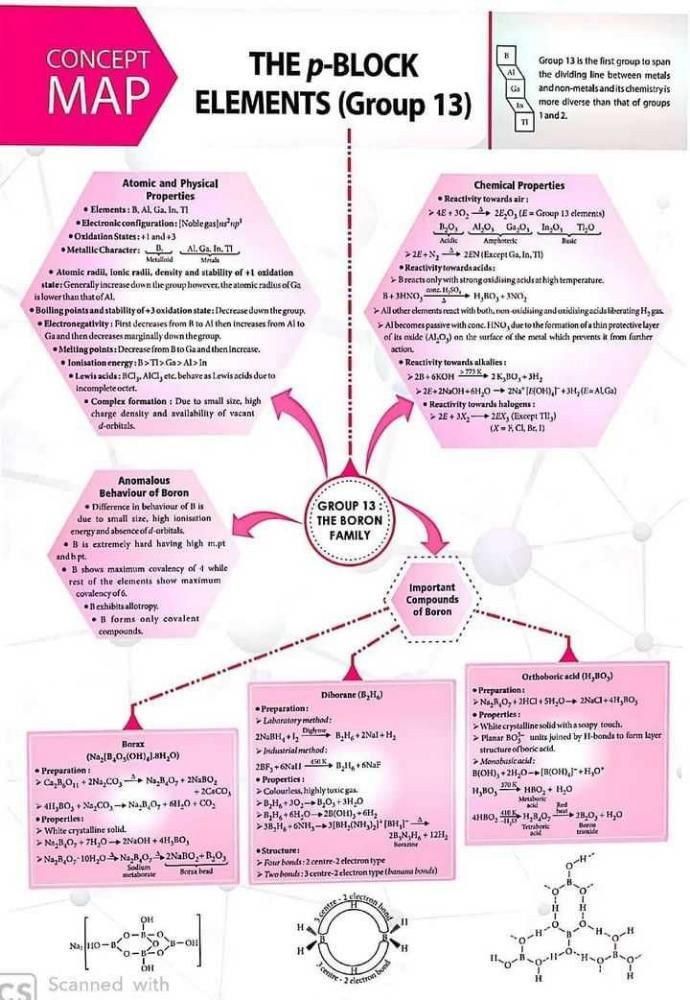
Community Answer
Concept map for p-block elements ( group 13 ) ?
Concept Map for p-Block Elements (Group 13)
p-block elements are those elements that have their valence electrons in the p-orbitals. Group 13 of the periodic table consists of boron (B), aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). These elements are characterized by their tendency to form a trivalent cation by losing three electrons. Here is a concept map that illustrates the properties and trends of Group 13 elements:
Physical Properties
- Boron is a non-metal, while the rest of the elements are metals.
- All elements in Group 13 have a low density and melting point.
- The boiling point increases down the group.
Chemical Properties
- Group 13 elements are characterized by their tendency to lose three electrons to form a trivalent cation.
- They react with non-metals to form covalent compounds.
- Aluminum is amphoteric, which means it can react with both acids and bases.
Boron
- Boron is a non-metal that has a high melting point and is a poor conductor of electricity.
- Boron forms covalent compounds with non-metals.
- Boron is used in the production of borosilicate glass and boron carbide.
Aluminum
- Aluminum is a highly reactive metal that has a low density and melting point.
- Aluminum has good thermal and electrical conductivity.
- Aluminum is used in the production of aircraft parts, beverage cans, and aluminum foil.
Gallium
- Gallium is a soft, silvery-white metal that has a low melting point.
- Gallium is used in the production of semiconductors, LEDs, and solar cells.
- Gallium has a unique property that allows it to melt in your hand.
Indium
- Indium is a soft, silvery-white metal that has a low melting point.
- Indium is used in the production of LCD screens, touch screens, and solar cells.
- Indium has a unique property that allows it to stick to glass.
Thallium
- Thallium is a soft, bluish-white metal that has a low melting point.
- Thallium is used in the production of infrared detection devices and medical imaging.
- Thallium is highly toxic and can cause serious health problems if ingested or inhaled.
In conclusion, Group 13 elements are characterized by their tendency to form a trivalent cation by losing three electrons. These elements have a variety of physical and chemical properties that make them useful in a variety of industries.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Concept map for p-block elements ( group 13 ) ?
Question Description
Concept map for p-block elements ( group 13 ) ? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Concept map for p-block elements ( group 13 ) ? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Concept map for p-block elements ( group 13 ) ?.
Concept map for p-block elements ( group 13 ) ? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Concept map for p-block elements ( group 13 ) ? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Concept map for p-block elements ( group 13 ) ?.
Solutions for Concept map for p-block elements ( group 13 ) ? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Concept map for p-block elements ( group 13 ) ? defined & explained in the simplest way possible. Besides giving the explanation of
Concept map for p-block elements ( group 13 ) ?, a detailed solution for Concept map for p-block elements ( group 13 ) ? has been provided alongside types of Concept map for p-block elements ( group 13 ) ? theory, EduRev gives you an
ample number of questions to practice Concept map for p-block elements ( group 13 ) ? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















