JEE Exam > JEE Questions > After understanding the assertion and reason,...
Start Learning for Free
After understanding the assertion and reason, choose the correct option.
Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.
Reason : The bonding MO is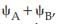 , which shows destructive interference of the combining electron waves.
, which shows destructive interference of the combining electron waves.
Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.
Reason : The bonding MO is
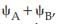 , which shows destructive interference of the combining electron waves.
, which shows destructive interference of the combining electron waves.- a)Assertion and reason are correct and reason is the correct explanation for the assertion.
- b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.
- c)Assertion is correct, reason is incorrect.
- d)Assertion is incorrect, reason is correct.
Correct answer is option 'C'. Can you explain this answer?

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer?
Question Description
After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer?.
After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer?.
Solutions for After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice After understanding the assertion and reason, choose the correct option.Assertion : In the bonding molecular orbital (MO) of H2, electron density is increased between the nuclei.Reason : The bonding MO is, which shows destructive interference of the combining electron waves.a)Assertion and reason are correct and reason is the correct explanation for the assertion.b)Assertion and reason are correct, but reason is not the correct explanation for the assertion.c)Assertion is correct, reason is incorrect.d)Assertion is incorrect, reason is correct.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























