Class 12 Exam > Class 12 Questions > Convert benzene to 1- bromo-3-ethylbenzene?
Start Learning for Free
Convert benzene to 1- bromo-3-ethylbenzene?
Most Upvoted Answer
Convert benzene to 1- bromo-3-ethylbenzene?
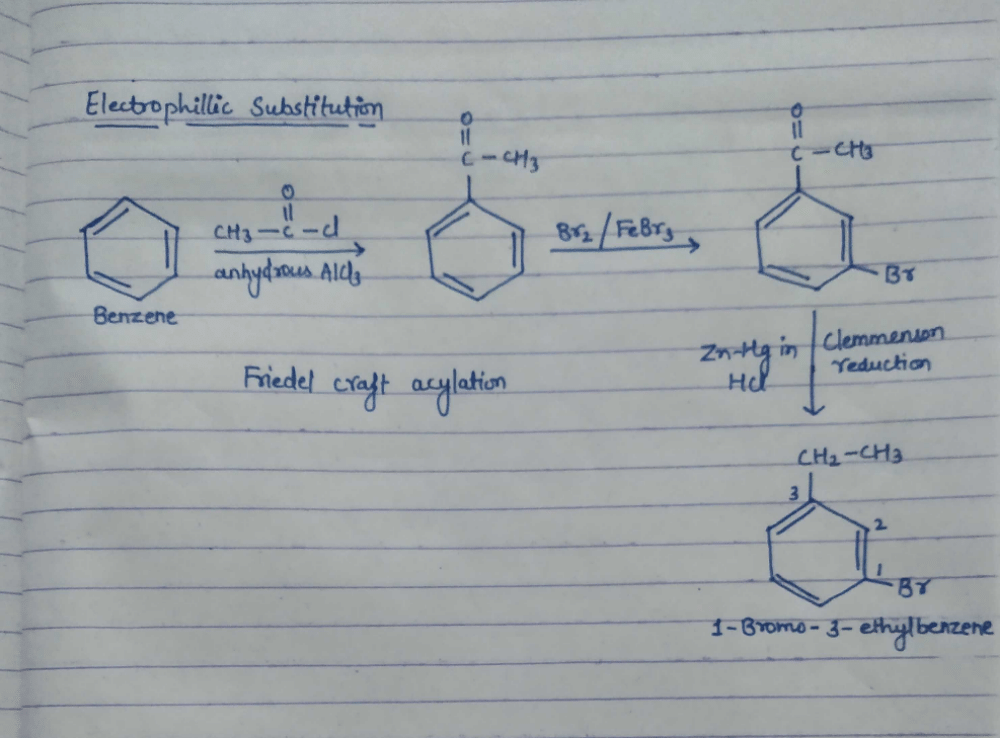
Community Answer
Convert benzene to 1- bromo-3-ethylbenzene?
**Conversion of Benzene to 1-Bromo-3-Ethylbenzene**
Benzene is a cyclic hydrocarbon with the molecular formula C6H6, while 1-Bromo-3-Ethylbenzene is an organic compound with the molecular formula C8H9Br. The conversion of benzene to 1-Bromo-3-Ethylbenzene involves introducing a bromine atom at the desired position and adding an ethyl group.
Here is a detailed explanation of the conversion process:
**Step 1: Bromination of Benzene**
The first step in the conversion is the bromination of benzene. This reaction is carried out by treating benzene with a brominating agent such as bromine (Br2) or a brominating reagent like N-bromosuccinimide (NBS) in the presence of a catalyst such as iron (Fe) or aluminum bromide (AlBr3). The reaction proceeds via electrophilic aromatic substitution. The bromine atom substitutes one of the hydrogen atoms on the benzene ring, resulting in the formation of bromobenzene.
**Step 2: Friedel-Crafts Alkylation**
The next step involves the addition of an ethyl group to the bromobenzene molecule. This reaction is known as Friedel-Crafts alkylation. It is carried out by treating bromobenzene with ethyl chloride (C2H5Cl) in the presence of a Lewis acid catalyst such as aluminum chloride (AlCl3). The reaction proceeds via electrophilic aromatic substitution, where the ethyl group replaces the hydrogen atom on the benzene ring, resulting in the formation of ethylbenzene.
**Step 3: Bromination of Ethylbenzene**
In this step, the bromination of the ethyl group on the ethylbenzene molecule is carried out. This reaction is similar to the bromination of benzene and involves the substitution of a hydrogen atom on the ethyl group with a bromine atom. The bromination can be achieved by treating ethylbenzene with bromine (Br2) or a brominating reagent such as N-bromosuccinimide (NBS) in the presence of a catalyst such as iron (Fe) or aluminum bromide (AlBr3). This reaction results in the formation of 1-Bromo-3-Ethylbenzene.
**Conclusion**
By following the above steps, benzene can be converted to 1-Bromo-3-Ethylbenzene. The bromination of benzene introduces a bromine atom, and the subsequent Friedel-Crafts alkylation and bromination of ethylbenzene add an ethyl group and a bromine atom, respectively. These steps result in the formation of 1-Bromo-3-Ethylbenzene, which is an important organic compound used in various applications.
Benzene is a cyclic hydrocarbon with the molecular formula C6H6, while 1-Bromo-3-Ethylbenzene is an organic compound with the molecular formula C8H9Br. The conversion of benzene to 1-Bromo-3-Ethylbenzene involves introducing a bromine atom at the desired position and adding an ethyl group.
Here is a detailed explanation of the conversion process:
**Step 1: Bromination of Benzene**
The first step in the conversion is the bromination of benzene. This reaction is carried out by treating benzene with a brominating agent such as bromine (Br2) or a brominating reagent like N-bromosuccinimide (NBS) in the presence of a catalyst such as iron (Fe) or aluminum bromide (AlBr3). The reaction proceeds via electrophilic aromatic substitution. The bromine atom substitutes one of the hydrogen atoms on the benzene ring, resulting in the formation of bromobenzene.
**Step 2: Friedel-Crafts Alkylation**
The next step involves the addition of an ethyl group to the bromobenzene molecule. This reaction is known as Friedel-Crafts alkylation. It is carried out by treating bromobenzene with ethyl chloride (C2H5Cl) in the presence of a Lewis acid catalyst such as aluminum chloride (AlCl3). The reaction proceeds via electrophilic aromatic substitution, where the ethyl group replaces the hydrogen atom on the benzene ring, resulting in the formation of ethylbenzene.
**Step 3: Bromination of Ethylbenzene**
In this step, the bromination of the ethyl group on the ethylbenzene molecule is carried out. This reaction is similar to the bromination of benzene and involves the substitution of a hydrogen atom on the ethyl group with a bromine atom. The bromination can be achieved by treating ethylbenzene with bromine (Br2) or a brominating reagent such as N-bromosuccinimide (NBS) in the presence of a catalyst such as iron (Fe) or aluminum bromide (AlBr3). This reaction results in the formation of 1-Bromo-3-Ethylbenzene.
**Conclusion**
By following the above steps, benzene can be converted to 1-Bromo-3-Ethylbenzene. The bromination of benzene introduces a bromine atom, and the subsequent Friedel-Crafts alkylation and bromination of ethylbenzene add an ethyl group and a bromine atom, respectively. These steps result in the formation of 1-Bromo-3-Ethylbenzene, which is an important organic compound used in various applications.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Convert benzene to 1- bromo-3-ethylbenzene?
Question Description
Convert benzene to 1- bromo-3-ethylbenzene? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Convert benzene to 1- bromo-3-ethylbenzene? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Convert benzene to 1- bromo-3-ethylbenzene?.
Convert benzene to 1- bromo-3-ethylbenzene? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Convert benzene to 1- bromo-3-ethylbenzene? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Convert benzene to 1- bromo-3-ethylbenzene?.
Solutions for Convert benzene to 1- bromo-3-ethylbenzene? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Convert benzene to 1- bromo-3-ethylbenzene? defined & explained in the simplest way possible. Besides giving the explanation of
Convert benzene to 1- bromo-3-ethylbenzene?, a detailed solution for Convert benzene to 1- bromo-3-ethylbenzene? has been provided alongside types of Convert benzene to 1- bromo-3-ethylbenzene? theory, EduRev gives you an
ample number of questions to practice Convert benzene to 1- bromo-3-ethylbenzene? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















