JEE Exam > JEE Questions > Taking Rydberg’s constant RH = 1.097 x ...
Start Learning for Free
Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum is
- a)2000 Å, 3000 Å
- b)1575 Å, 2960 Å
- c)6529 Å, 4280 Å
- d)6552 Å, 4863 Å
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Taking Rydberg’s constant RH = 1.097 x 107m, first and second wa...
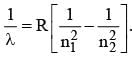
For first wavelength, n1 = 2, n2 = 3
⇒ λ1 = 6563 Å. For second wavelength, n1 = 2, n2 = 4
⇒ λ2 = 4861 Å
Most Upvoted Answer
Taking Rydberg’s constant RH = 1.097 x 107m, first and second wa...
Taking Rydberg refers to the process of a neutral atom absorbing energy and transitioning to a Rydberg state. Rydberg states are highly excited states of an atom, where one or more electrons are in high principal quantum number orbitals. These states are typically far away from the ground state and have very large radii.
The process of taking Rydberg involves supplying energy to an atom, typically through the absorption of photons or collision with other particles. When an atom absorbs enough energy, one of its electrons can be promoted to a higher energy level and occupy a Rydberg state.
The energy required to take an atom to a Rydberg state is typically on the order of electron volts (eV) or higher. This energy can come from various sources such as lasers, electric fields, or collisions with other particles. Once in a Rydberg state, the atom can exhibit unique properties and behaviors due to the large size and energy of the electron orbitals.
Rydberg states have been extensively studied in atomic physics and have applications in fields such as quantum optics, quantum computing, and spectroscopy. They offer a rich playground for exploring quantum phenomena and can be manipulated and controlled using external fields and interactions.
In summary, taking Rydberg refers to the process of exciting an atom to a highly excited state with a high principal quantum number, known as a Rydberg state. This process can be achieved by supplying energy to the atom through various means, leading to unique behaviors and applications in atomic physics.
The process of taking Rydberg involves supplying energy to an atom, typically through the absorption of photons or collision with other particles. When an atom absorbs enough energy, one of its electrons can be promoted to a higher energy level and occupy a Rydberg state.
The energy required to take an atom to a Rydberg state is typically on the order of electron volts (eV) or higher. This energy can come from various sources such as lasers, electric fields, or collisions with other particles. Once in a Rydberg state, the atom can exhibit unique properties and behaviors due to the large size and energy of the electron orbitals.
Rydberg states have been extensively studied in atomic physics and have applications in fields such as quantum optics, quantum computing, and spectroscopy. They offer a rich playground for exploring quantum phenomena and can be manipulated and controlled using external fields and interactions.
In summary, taking Rydberg refers to the process of exciting an atom to a highly excited state with a high principal quantum number, known as a Rydberg state. This process can be achieved by supplying energy to the atom through various means, leading to unique behaviors and applications in atomic physics.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer?
Question Description
Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer?.
Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Taking Rydberg’s constant RH = 1.097 x 107m, first and second wavelength of Balmer series in hydrogen spectrum isa)2000 Å, 3000 Åb)1575 Å, 2960 Åc)6529 Å, 4280 Åd)6552 Å, 4863 ÅCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























