Class 11 Exam > Class 11 Questions > In the following question, a statement of As...
Start Learning for Free
In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.
Choose the correct option out of the choices given below each question.
Assertion (A): Ionic bonds are always stronger than covalent bonds.
Reason(B): They break only when bombarded with electrons.
- a)Both A and R are true and R is the correct explanation of A.
- b)Both A and R are true but R is not the correct explanation of A.
- c)A is true but R is false.
- d)Both A and R are false.
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
In the following question, a statement of Assertion (A) followed by a...
Ionic bond involves complete transfer of electrons as there occurs formation of ions called cation and anion therefore, there exists a huge electrostatic force of attraction which makes it a strong bond. For example, sodium chloride is formed by an ionic bond between sodium and chloride ions.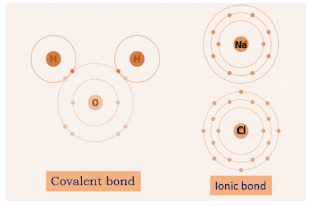
View all questions of this test
Covalent bond involves sharing of two or more outer shell electrons that can hold all biomolecules together. Shared electrons are difficult to give away because two elements together share the electrons and make the bond stronger. For example, water molecules have covalent bonds in them.
We know that bond strength is inversely proportional to bond length. Bond length of ionic bonds is less as compared to that of covalent bonds. So we can say that ionic bonds are stronger than covalent bonds. Ionic bond is formed when there is small ionisation whereas covalent bonding occurs when polarisation degree is high.

Since the presence of coulombic attraction between oppositely charged ions in ionic bonding leads to its higher stability, it is stronger than the covalent bonding. Hence, the correct option is (C).
Most Upvoted Answer
In the following question, a statement of Assertion (A) followed by a...
Explanation:
Assertion (A):
- Ionic bonds are not always stronger than covalent bonds.
- The strength of a bond depends on various factors such as the nature of the atoms involved, the distance between them, and the presence of any external forces.
Reason (R):
- Ionic bonds do not break only when bombarded with electrons.
- Ionic bonds are formed by the transfer of electrons from one atom to another, resulting in the attraction between oppositely charged ions.
- They are relatively strong bonds but can still be broken under certain conditions.
Therefore, the correct option is:
c) A is true but R is false.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer?
Question Description
In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer?.
In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer?.
Solutions for In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given.Choose the correct option out of the choices given below each question.Assertion (A): Ionic bonds are always stronger than covalent bonds.Reason(B): They break only when bombarded with electrons.a)Both A and R are true and R is the correct explanation of A.b)Both A and R are true but R is not the correct explanation of A.c)A is true but R is false.d)Both A and R are false.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























