Class 11 Exam > Class 11 Questions > Maxwell Distribution Function In a given mas...
Start Learning for Free
Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.
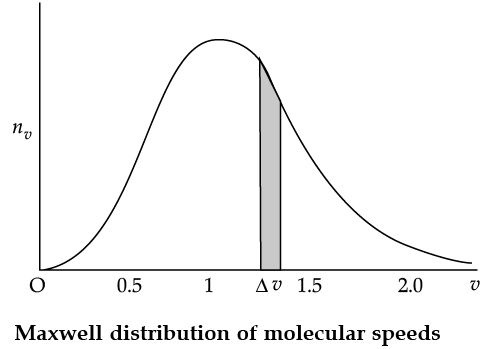
The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integral
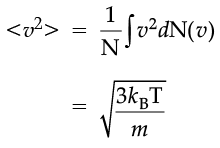
which agrees with the result derived from more elementary considerations.
Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given by
- a)dN(v) = 4p Na3 ebv2 v2 dv
- b)dN(v) = 4p Na3 e–bv2v dv
- c)dN(v) = 4 Na3 e–bv2 v2 dv
- d)dN(v) = 4p Na3 e–bv2 v2 dv
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Maxwell Distribution Function In a given mass of gas, the velocities ...
The molecular speed distribution gives the number of molecules between the speeds v and v + dv. D
View all questions of this test
dN(v) = 4p Na3 e–bv2 v2 dv = nvdv.
This is called Maxwell distribution.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer?
Question Description
Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer?.
Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Maxwell Distribution Function In a given mass of gas, the velocities of all molecules are not the same, even when bulk parameters like pressure, volume and temperature are fixed. Collisions change the direction and the speed of molecules. However in a state of equilibrium, the distribution of speeds is constant or fixed. Distributions are very important and useful when dealing with systems containing large number of objects. As an example consider the ages of different persons in a city. It is not feasible to deal with the age of each individual. We can divide the people into groups: children up to age 20 years, adults between ages of 20 and 60, old people above 60. If we want more detailed information we can choose smaller intervals, 0–1, 1–2,..., 99– 100 of age groups. When the size of the interval becomes smaller, say half year, the number of persons in the interval will also reduce, roughly half the original number in the one year interval. The number of persons dN(x) in the age interval x and x + dx is proportional to dx or dN(x) = nx dx. We have used nx to denote the number of persons at the value of x. In a similar way the molecular speed distribution gives the number of molecules between the speeds v and v + dv. dN(v) = 4p N a3 e–bv2v2dv = nvdv. This is called Maxwell distribution.The plot of nv against v is shown in the figure. The fraction of the molecules with speeds v and v + dv is equal to the area of the strip shown. The average of any quantity like v2 is defined by the integralwhich agrees with the result derived from more elementary considerations.Q. The molecular speed distribution of number of molecules between the speeds v and v+ dv is given bya)dN(v) = 4p Na3 ebv2 v2 dvb)dN(v) = 4p Na3 e–bv2v dvc)dN(v) = 4 Na3 e–bv2 v2 dvd)dN(v) = 4p Na3 e–bv2 v2 dvCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























