Class 11 Exam > Class 11 Questions > Attempt All sub parts from each question.If ...
Start Learning for Free
Attempt All sub parts from each question.
If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.
It does this by following the steps below:
• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.
• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.
• The coolant expands and it cools down below the temperature inside the refrigerator.
• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.
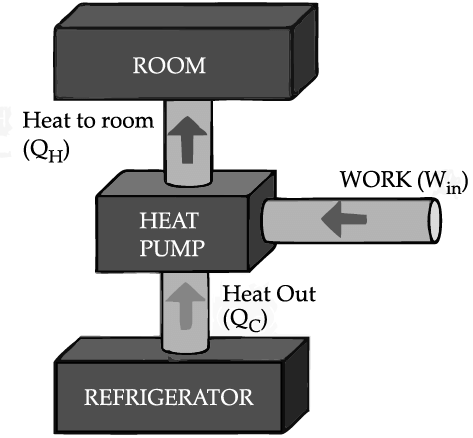
So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.
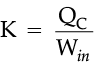
The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.
Q. Which of the following statement is true?- a)less work is being done to cool down the refrigerator, higher the coefficient of performance
- b)More work is being done to cool down the refrigerator, higher the coefficient of performance
- c)coefficient of performance has no relation with the work
- d)coefficient of performance depends on ambient temperature
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Attempt All sub parts from each question.If we turn on our electric o...
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.
View all questions of this test

The higher this value is the better the refrigerator, because it means that less work is being done to cool down the refrigerator. For higher value of coefficient of performance, Win should be less.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer?
Question Description
Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer?.
Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Attempt All sub parts from each question.If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.It does this by following the steps below:• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.• The coolant expands and it cools down below the temperature inside the refrigerator.• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.Q. Which of the following statement is true?a)less work is being done to cool down the refrigerator, higher the coefficient of performanceb)More work is being done to cool down the refrigerator, higher the coefficient of performancec)coefficient of performance has no relation with the workd)coefficient of performance depends on ambient temperatureCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























