Class 12 Exam > Class 12 Questions > Read the passage given below and answer the ...
Start Learning for Free
Read the passage given below and answer the following questions:
Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :
Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.
Reason (R): The electron density is more at ortho and para positions.
- a)Assertion and reason both are correct statements and reason is correct explanation for assertion.
- b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.
- c)Assertion is correct statement but reason is wrong statement.
- d)Assertion is wrong statement but reason is correct statement.
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Read the passage given below and answer the following questions:Benze...
In aniline, the electron density is more at ortho and para positions than meta position, so, the substitution mainly takes place at ortho and para positions.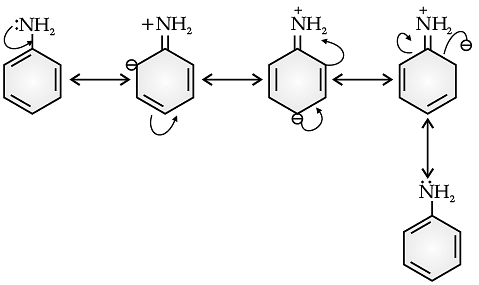

The above resonating structures of aniline show more electron density at the ortho and para positions.
Free Test
FREE
| Start Free Test |
Community Answer
Read the passage given below and answer the following questions:Benze...
Explanation:
Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.
- This statement is correct because aniline is highly activated due to the lone pair of nitrogen being shared with the benzene ring. This results in an increase in the electron density on the ring, making it more susceptible to electrophilic attack. As a result, substitution mainly occurs at the ortho and para positions where the electron density is higher.
Reason (R): The electron density is more at ortho and para positions.
- This statement is incorrect. The electron density is actually more at the ortho and para positions due to the sharing of the lone pair of nitrogen with the benzene ring. This increased electron density makes these positions more reactive and hence more prone to substitution reactions.
Therefore, the correct answer is:
c) Assertion is a correct statement, but the reason is a wrong statement.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer?
Question Description
Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer?.
Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Read the passage given below and answer the following questions:Benzene ring in aniline is highly activated. This is due to the sharing of lone pair of nitrogen with the ring which results in increase in the electron density on the ring and hence facilitates the electrophilic attack. The substitution mainly takes place at ortho and para positions because electron density is more at ortho and para positions. On reaction with aqueous bromine all the ortho and para positions get substituted resulting in the formation of 2,4,6-tribromoaniline. To get a monobromo compound, the amino group is acetylated before bromination. After bromination, the bromoacetanilide is acid hydrolysed to give the desired halogenated amine.In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices :Assertion (A): In aniline, the substitution mainly takes place at ortho and para positions.Reason (R): The electron density is more at ortho and para positions.a)Assertion and reason both are correct statements and reason is correct explanation for assertion.b)Assertion and reason both are correct statements but reason is not correct explanation for assertion.c)Assertion is correct statement but reason is wrong statement.d)Assertion is wrong statement but reason is correct statement.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















