Class 12 Exam > Class 12 Questions > Read the passage given below and answer the ...
Start Learning for Free
Read the passage given below and answer the following questions:
Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.
Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?
- a)Fehling’s test
- b)Benedict’s test
- c)Iodoform test
- d)Hinsberg reagent
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Read the passage given below and answer the following questions:Aldeh...
On heating an aldehyde with Fehling’s reagent, a reddish brown precipitate is obtained. Aldehydes are oxidised to corresponding carboxylate anion. Aromatic aldehydes do not respond to this test.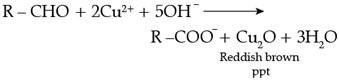
View all questions of this test
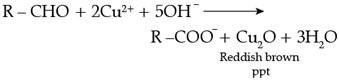
Most Upvoted Answer
Read the passage given below and answer the following questions:Aldeh...
How to distinguish between aliphatic aldehydes and aromatic aldehydes?
To distinguish between aliphatic aldehydes and aromatic aldehydes, various tests can be performed. One such test is the Fehling's test.
Fehling's test:
Fehling's test is used to distinguish between aliphatic aldehydes and aromatic aldehydes based on their reducing properties. It involves the reaction of the aldehyde with Fehling's solution, which is a mixture of copper sulfate and sodium hydroxide.
Procedure:
1. Take a small amount of the aldehyde to be tested in a test tube.
2. Add an equal amount of Fehling's solution to the test tube.
3. Heat the mixture gently by placing the test tube in a water bath or directly heating it.
4. Observe the color change in the solution.
Observations:
- Aliphatic aldehydes, such as formaldehyde or acetaldehyde, give a positive test and produce a red precipitate of copper(I) oxide.
- Aromatic aldehydes, such as benzaldehyde, do not give a positive test and the solution remains blue.
Explanation:
The Fehling's test relies on the reducing properties of aldehydes. Aliphatic aldehydes contain a terminal carbonyl group, which can be oxidized to a carboxylic acid. During the test, the aldehyde reduces the copper(II) ions present in Fehling's solution to copper(I) ions, resulting in the formation of a red precipitate of copper(I) oxide.
Aromatic aldehydes, on the other hand, do not readily undergo oxidation due to the presence of electron-withdrawing groups attached to the aromatic ring. As a result, they do not give a positive Fehling's test and the solution remains blue.
Conclusion:
By performing the Fehling's test, one can distinguish between aliphatic aldehydes and aromatic aldehydes based on their ability to reduce Fehling's solution and produce a red precipitate. Aliphatic aldehydes give a positive test, while aromatic aldehydes do not.
To distinguish between aliphatic aldehydes and aromatic aldehydes, various tests can be performed. One such test is the Fehling's test.
Fehling's test:
Fehling's test is used to distinguish between aliphatic aldehydes and aromatic aldehydes based on their reducing properties. It involves the reaction of the aldehyde with Fehling's solution, which is a mixture of copper sulfate and sodium hydroxide.
Procedure:
1. Take a small amount of the aldehyde to be tested in a test tube.
2. Add an equal amount of Fehling's solution to the test tube.
3. Heat the mixture gently by placing the test tube in a water bath or directly heating it.
4. Observe the color change in the solution.
Observations:
- Aliphatic aldehydes, such as formaldehyde or acetaldehyde, give a positive test and produce a red precipitate of copper(I) oxide.
- Aromatic aldehydes, such as benzaldehyde, do not give a positive test and the solution remains blue.
Explanation:
The Fehling's test relies on the reducing properties of aldehydes. Aliphatic aldehydes contain a terminal carbonyl group, which can be oxidized to a carboxylic acid. During the test, the aldehyde reduces the copper(II) ions present in Fehling's solution to copper(I) ions, resulting in the formation of a red precipitate of copper(I) oxide.
Aromatic aldehydes, on the other hand, do not readily undergo oxidation due to the presence of electron-withdrawing groups attached to the aromatic ring. As a result, they do not give a positive Fehling's test and the solution remains blue.
Conclusion:
By performing the Fehling's test, one can distinguish between aliphatic aldehydes and aromatic aldehydes based on their ability to reduce Fehling's solution and produce a red precipitate. Aliphatic aldehydes give a positive test, while aromatic aldehydes do not.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer?
Question Description
Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer?.
Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Read the passage given below and answer the following questions:Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?a)Fehling’s testb)Benedict’s testc)Iodoform testd)Hinsberg reagentCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















